Latest Research
- 2016.07.01
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
Towards the Control of Enzyme Activity by a Molecule of your Choice
〜Development of Antibody-fused Enzymes〜
Introduction
There is a class of enzymes in nature called an allosteric enzyme, whose activity is controlled by their substrate or other molecule(s) called ligand(s). The activity of an allosteric enzyme is often controlled by a fine-tuning of its multimeric enzyme structure, and has been regarded hard to achieve in artificial way. However, recently Ostermeier et al. reported construction of fusion proteins that are made of a certain sugar binding protein (Maltose Binding Protein, MBP) and a hydrolytic enzyme involved in antibiotic resistance (β-lactamase, BLA) whose activity is effectively controlled by the binding of MBP, after rounds of molecular evolution.1,2) This work has its value by showing that the fusion of two unrelated of monomeric enzymes leads to an enzyme that manifests allosteric-like activity. However, the obtained proteins could respond only to maltose or similar sugars, which might not make this approach versatile enough. This time, we tried to expand the approach by using the binding domain of antibody, to make more versatile allosteric enzymes that respond to more variety of ligands.
1. A fusion protein of antibody variable region and circularly permuted β-lactamase3)
First, we made a fusion protein of antibody variable region Fv that binds antigen, and a circularly permuted (Note 1) β-lactamase as reported previously (Fv-cpBLA)(Fig. 1).
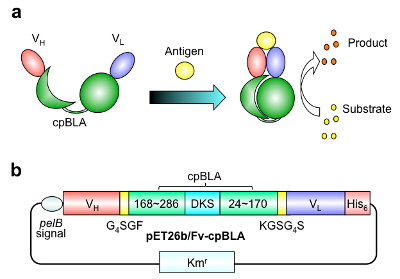
Fig. 1 First generation enzyme Fv-cpBLA (a) Schematic structure and (b) gene structure. (Copyright American Chemical Society)
As antibodies, we used two for small molecule antigens, namely, the C-terminal peptide of osteocalcin (BGP) that is used as a diagnostic marker for bone diseases, and imidacloprid (ICP) that is one of neonicotinoid pesticides whose toxicity to bees and birds is under great concern worldwide. It is also worth noting that the antigen binding domain of these two antibodies Fv is known to be stabilized by the binding of antigen. In other words, the interaction between the two domain of Fv, VH and VL, is markedly strengthened by antigen binding. Hence, we expected that Fv-cpBLA is stabilized by the antigen binding at its Fv, and lead to the stabilization and activation of cpBLA.
When we expressed and purified Fv-cpBLA for BGP using E. coli and added with an antigen BGP-C7, significant increase in BLA activity was observed (Fig. 2a). On the other hand, non-relevant molecules ICP or TCP did not show such effect (Fig. 2b),Also, wild-type BLA without Fv did not respond to BGP-C7, showing its antigen specific activation (Fig. 2c)。
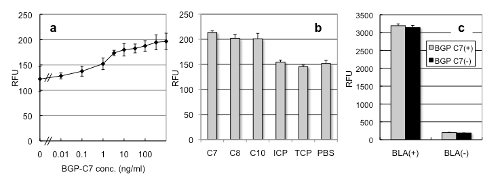
Fig. 2 BGP specific Fv-cpBLA
(a) Antigen-dependent enzyme activity measured by fluorescent substrate.
(b) Specificity of activation. 8, C10 are fragments of BGP C-terminus.
(c) Negligible effect of BGP-C7 on the wild-type BLA.
Moreover, similarly prepared Fv-cpBLA for ICP also showed ICP binding (Fig. 3a),and ICP (and similar pesticide TCP)-dependent BLA activity (Fig. 3b, c). Moreover, E. coli expressing Fv-cpBLA for ICP showed antigen-dependent growth property, when grown in the presence of ampicillin, a b-lactam antibiotic (Fig. 3d). Probably,the elevated BLA activity of Fv-cpBLA lead to increased ampicillin degradation and bacterial growth.
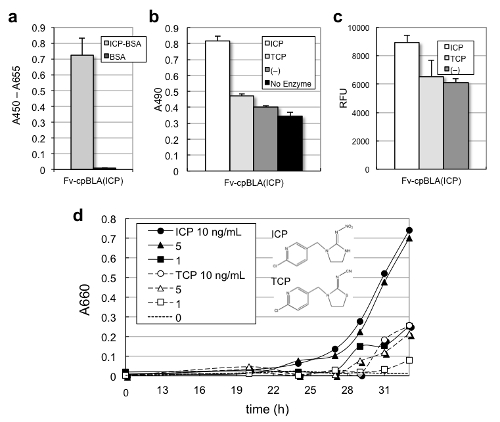
Fig. 3 Activation of Fv-cpBLA for neonicotinoid pesticides.
(a) Antigen-binding activity measured by ELISA.
(b) Activity measured with colorimetric substrate nitrocefin.
(c) The same with fluorescent substrate fluorocillin.
(d) Antigen-dependent cellular growth.
2. Creation of circularly permuted VH/VL (Clampbody)
Although the results so far are good as a proof of principle also attaining high sensitivity and selectivity, the extent of activation was not so remarkable. So next we tried further engineering of antibody Fv domain to attain higher response. To this end, further circular permutations were introduced. The two domains of Fv, VH and VL, were each circularly permuted by connecting their original N-C termini by a flexible linker, and the new termini were made at the loop situating on the opposite side of antigen binding site near the VH/VL interface. Then we named this combination (cpVH and cpVL) as a new binding unit Clampbody (Cbody).
To test the antigen binding activity of Clampbody, first we connected cpVH and cpVL by a short linker to make the gene for a single chain Clampbody (sc-Cbody) (Fig. 4a). Then the second generation fusion protein Cbody-cpBLA was made by fusing cpBLA4) to the termini of cpVH and cpVL (Fig. 4b). The proteins were expressed in the insoluble fraction of E. coli, refolded, and purified to be analyzed for their activity as follows.
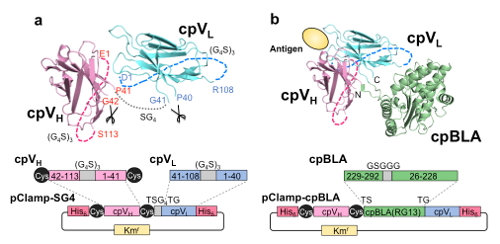
Fig. 4 Scheme of Clampbody (sc-Cbody) (a) and Cbody-cpBLA (b). A Cys pair was introduced to stabilize cpVH by a SS bond. (Copyright American Chemical Society)
3. Activation of Clampbody-fused enzyme 5)
As before, the antigen binding activity of Clampbody was measured by ELISA. As shown in Fig. 5, both sc-Cbody (a) and Cbody-cpBLA (b) were confirmed to retain antigen BGP peptide binding activity.
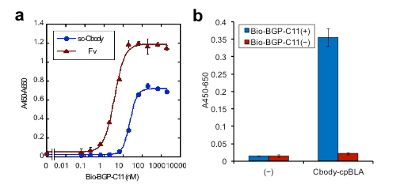
Fig. 5 Antigen binding ELISA for (a) single chain Clampbody and (b) Cbody-cpBLA.
Next, the BLA activity of Cbody-cpBLA was evaluated at several conditions (Fig. 6). When it was measured in phosphate buffered saline (PBS), the increase in activity in the presence of 1 µM antigen was not so remarkable (a). However, that in the presence of weak denaturant (b) or detergent (c) showed more remarkable increase. When the activity was measured at the condition of (c), a clear antigen dose dependency in BLA activity (fluorescence increase rate) was observed up to 4.7-fold, with the detection limit less than 1 nM, which is less than blood BGP concentration in healthy individuals. Hence, by using Clampbody, we could get a superior antigen-dependent enzyme.
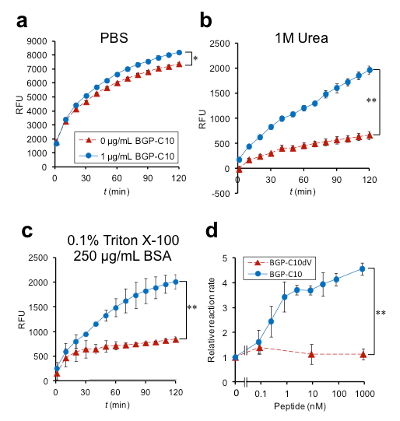
Fig. 6 Antigen-dependent activity of second generation enzyme Cbody-cpBLA. (a)-(c) shows the fluorescence time course under the buffer condition as specified, while (d) shows the dose-dependency in BLA activity at the condition in (c).
4. Summary
Recent report indicated a model of allosteric regulation by protein stabilization, in addition to conformational change (ensemble model)6). The results obtained this time is considered to be explained by this model. Also, not only denaturant but also detergent was found effective to control its activity. Hopefully, these observations will contribute to further understanding of allosteric regulation of proteins, and also creation of better biosensors with more robust antigen dependent enzyme activity.
(Note 1) Circular permutation : a mutation of proteins whose terminal position is moved to another position, while original termini are connected. The structure and activity is retained. Also found in natural proteins, probably due to gene duplication and deletion.
References
1) Guntas, G., Mansell, T. J., Kim, J. R., et al.: Proc. Natl. Acad. Sci. USA, 102, 11224-11229 (2005)
2) Ostermeier, M.: Protein Eng Des Sel. , 18, 359-364 (2005)
3) Kojima, M., Iwai, H., Dong, J., et al.: Bioconj. Chem., 22, 633-641 (2011)
4) Ke, W., Laurent, A. H., Armstrong, M. D., et al.: Plos One, 7, e39168 (2012)
5) Iwai, H., Kojima-Misaizu, M., Dong, J., et al.: Bioconj. Chem., 27, 868-873 (2016)
6) Choi, J. H., Laurent, A. H., Hilser, V. J., et al.: Nat Commun, 6, 6968 (2015)




