Latest Research
- 2017.08.01
How to Make "Molecular Peanuts"
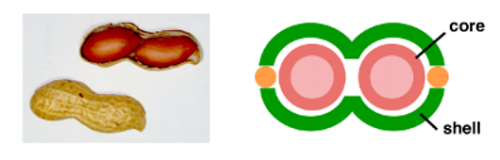
Mimicking the fascinating motion of animals and shapes of plants using molecules is an ongoing challenge for synthetic chemists. Multilayered bio-structures such as flowers, fruits, and seeds are so complicated that chemical mimicry of such structures is extremely difficult. Peanuts are well known seeds bearing a couple of beans surrounded by a pod (Figure 1, left). However, imitation of the unique core-shell structure has not been accomplished so far on the nanoscale. Here, Yazaki, Chand, and Yoshizawa et al. report the synthesis of peanut-shaped nanostructures constructed of polyaromatic frameworks (Figure 1, right).[1] Our synthetic approach to the molecular peanuts is the use of both coordination bonds and π-stacking interactions.
Figure 1 Photograph of a peanut and design of a molecular peanut.
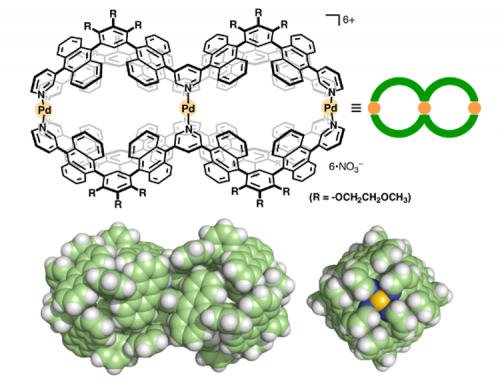
We prepared W-shaped polyaromatic ligand 1 from bromoanthracene by six-step reactions including Negishi and Suzuki-Miyaura cross-couplings (Figure 2, top). It should be noted that ligand 1 exists as a mixture of ten stereoisomers due to restricted rotation about the aromatic-aromatic bonds (Figure 2, bottom).
Figure 2 W-shaped ligand 1 and its stereoisomers (R = -OCH3).
When an isomeric mixture of ligand 1 was combined with Pd(II) ion in a 4:3 ratio in DMSO at elevated temperature, double capsule 2 was quantitatively formed through multiple coordination bonds (Figure 3, top). Structural evidence of the double capsule was provided by single crystal X-ray diffraction analysis (in collaboration with Rigaku Co.). The dumbbell-shaped structure has two identical, spherical cavities with a diameter of ~1 nm, each fully surrounded by the eight anthracene panels (Figure 3, bottom).
Figure 3 Double capsule 2 and its crystal structure (R = -H).
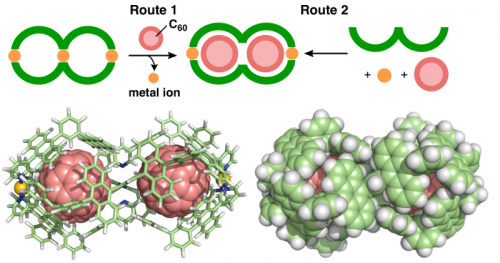
The conversion from double capsule 2 to molecular peanuts occurred quantitatively upon treatment with various fullerenes (e.g., C60, C70, and Sc3N@C80). For example, when C60 powder suspended in a solution of 2 was heated for several hours, molecular peanut 3 including two molecules of C60 was obtained through multiple host-guest π-stacking interactions and the release of the central Pd(II) ion (Figure 4, synthetic route 1). The optimized structure shows a peanut-shaped nanostructure, where two C60 "beans" accommodated in the cavity are fully covered with the dumbbell-like "pod" (Figure 4, bottom). The product size is estimated to be ~3 nm. It is noteworthy that the molecular peanut could be also obtained in one step by mixing ligand 1, Pd(II) ion, and C60 (Figure 4, synthetic route 2).
Figure 4 Formation of molecular peanut 3 and its optimized structure.
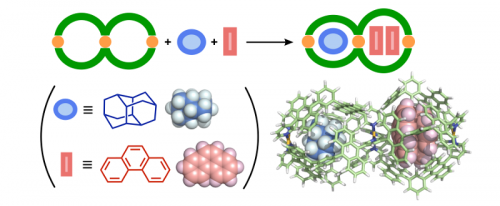
We also observed the selective heteroleptic encapsulation of aliphatic and aromatic molecules by double capsule 2. Mixing spherical diamantane and planar phenanthrene with 2 in an aqueous solution gave rise to a 1:1:2 host-guest-guest' complex in nearly quantitative yield (Figure 5). This unusual encapsulation arises from the cooperative changes in volume of the linked cavities upon guest encapsulation.
Figure 5 Heteroleptic encapsulation by double capsule 2 and its optimized structure.
In summary, we have created peanut-shaped nanostructures through stepwise and one-pot self-assembly. The unusual core-shell nanostructures quantitatively form through multiple coordination bonds and π-stacking interactions. This synthetic strategy could provide wide-ranging utilities for the facile preparation of various complex bio-structures.[2-6]
References
[1] K. Yazaki, M. Akita, S. Prusty, D. K. Chand, T. Kikuchi,H. Sato,
M. Yoshizawa*, Nature Commun., 2017, 8, 15914
(link: https://www.nature.com/articles/ncomms15914).
Our recent reports
[2] S. Matsuno, M. Yamashina, Y. Sei, M. Akita, A. Kuzume,K. Yamamoto, M. Yoshizawa*, Nature Commun., 2017, 8, in press.
[3] K. Kurihara, K. Yazaki, M. Akita, M. Yoshizawa*, Angew. Chem. Int. Ed., 2017, 56, in press.
[4] K. Jono, A. Suzuki, M. Akita, K. Albrecht, K. Yamamoto, M. Yoshizawa*, Angew. Chem. Int. Ed., 2017, 56, 3570-3574.
[5] M. Kishimoto, K. Kondo, M. Akita, M. Yoshizawa*, Chem. Commun., 2017, 53, 1425-1428.
[6] M. Yoshizawa*, M. Yamashina, Chem. Lett., 2017, 46, 163-171.