Latest Research
- 2017.09.08
- Fukushima-Shoji Group
Novel boron-mediated benzannulation reaction of alkynes enabling the facile synthesis of extended π-conjugated systems
π-Conjugated molecules and polymers constitute an important class of materials that are useful for a wide variety of applications including organic electronics. Efficient synthetic methodology for the π-conjugated materials are always important, since their electronic, opt-electronic, and physical properties are largely dependent on how the π-conjugated systems are interconnected. However, even with cross-coupling reactions, which are one of the most powerful methods to construct π-conjugated architectures, multi-step and tedious synthetic procedures are often required to synthesize extended π-conjugated molecules.
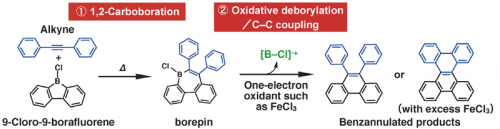
We have found that a certain boron compound, that is, 9-chloro-9-borafluorene, mediates sequential C-C bond forming reactions, which enables the facile benzannulation of alkynes to form extended π-conjugated molecules (Fig. 1, ref 1). The first step is the alkyne insertion into a B-C bond of borafluorene (1,2-carboboration), which gives borepin, a boron-containing seven-membered-ring compound. The second step, oxdative deborylation/C-C coupling, is caused by a one-electron oxidation of the borepin derivative, affording a phenanthrene framework. The second-step reaction is striking in that, considering the material balance of the reaction, a low-valent cationic boron species such as [B-Cl]+• should be generated from the borepin radical cation (Fig. 1). Such low-valent cationic species of boron usually represents a very high-energy state (refs 2,3).
Fig. 1. Boron-mediated sequential C-C coupling reactions.
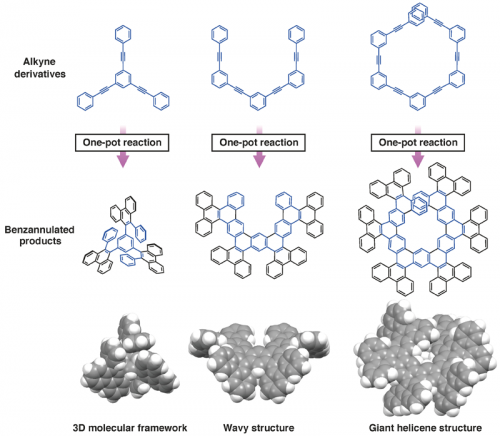
The sequential 1,2-carbobration and oxidative deborylation/C-C coupling reactions can be performed in a one-pot fashion, allowing the facile benzannulation of various functionalized alkyne derivatives. Inexpensive oxidants such as FeCl3 and MnO2 can be used in the second step. Figure 2 shows examples of the π-extended molecules that can be obtained by the borafluorene-mediated benzannulation reaction of alkyne derivatives. It is noteworthy that such largely π-extended molecules with a three-dimensional molecular framework can be obtained without transition-metal catalysts.
Fig. 2. Examples of π-extended molecules that can be obtained by the novel boron-mediated benzanuulation reaction of alkynes.
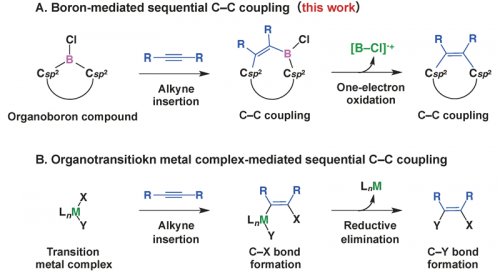
Organotransition metal complexes often allow the insertion of unsaturated compounds into the metal-carbon bonds, and in some cases, the resulting complexes then undergo elimination of the metal moiety to cause cross-coupling (Fig. 3B). This reaction sequence has set the chemistry of transition metals apart from that of main group elements. However, one might find some analogy to the sequential 1,2-carboboration of alkynes by borafluorene and the subsequent oxidative deborylation/C-C coupling (Fig. 3A); this unprecedented reactivity may be interpreted in terms of boron behaving like a transition metal in C-C coupling.
Fig. 3. Comparison between (A) the boron-mediated sequential C-C coupling reactions and (B) typical reactivity of organotransition metal complexes.
The unprecedented reaction of organoboron compounds (Fig. 1) allows the facile transformation of alkynes into non-planar, curved, and three-dimensional π-conjugated molecules. Such π-conjugated systems are expected to show dynamic conformational changes, unique optical and chiroptical properties, and particular assembling behaviors, which may be beneficial for applications in organic electronics. On the basis of these findings, 9-Chloro-9-borafluorene, a key organoboron compound in this synthetic protocol, became commercially available (ref. 4). Further studies aiming at experimentally determining the reaction mechanism of the oxidative deborylation/C-C coupling (Fig. 1) is now underway.
[1] Y. Shoji, N. Tanaka, S. Muranaka, N. Shigeno, H. Sugiyama, K. Takenouchi, F. Hajjaj, T. Fukushima, Nature Commun. 2016, 7, 12704.
[2] Y. Shoji, N. Tanaka, K. Mikami, M. Uchiyama, T. Fukushima, Nature Chem. 2014, 6, 498.
[3] The CLS Website, Latest Research "Development of Boron-based Molecular Super Lewis Acid" (http://www.res.titech.ac.jp/english/news/reserch/
development_of_boron-based_molecular_super_lewis_acid.html)
[4] The TCI Website, "Borafluorene for the Efficient Synthesis of Extended π-Conjugated Systems" (http://www.tcichemicals.com/pdf/FF056E.pdf)