Latest Research
- 2017.10.02
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
Tailor-made biosensor to color the molecular world
To clarify the mechanisms for maintaining homeostasis, cell growth and cause of disease in our body, it is important to understand how molecules, such as proteins, lipids, ions, metabolites etc., distribute, translocate and interact with each other. Driven by this motivation, we developed a new class of genetically-encoded antibody-based biosensor for visualizing the behavior of a molecule of interest in living cellsref1.
In general, many molecules in living cells do not have color or fluorescence, therefore it is not easy to visualize or detect them at high spatiotemporal resolution. To make these molecules optically visible, various fluorescent biosensors have been developed which could bind to molecules and report their dynamics by changing fluorescence signal. In particular, biosensors utilizing fluorescent proteins are frequently exploited for live cell imaging because they can be easily expressed in living cells and organism. These fluorescent protein biosensors have been created by employing a known binding protein for molecular recognition. However, if the binding protein of the molecule of interest is unknown or does not exist in nature, it is difficult to develop the sensor. Therefore, by applying an antibody as the molecular recognition domain, we aspire to develop biosensors for myriad of molecules (Fig. 1).
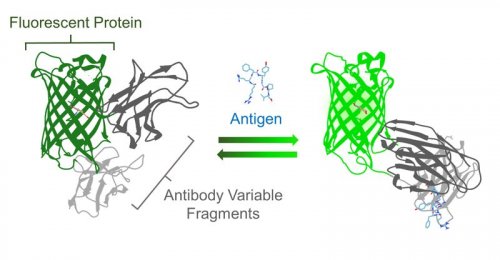 Figure 1ref1
Figure 1ref1
Schematic diagram of the Flashbody. (©American Chemical Society)
The prototype of antibody-based biosensor was constructed by fusing the heavy chain (VH) and the light chain (VL) of the variable region of antibody to the N-terminus and the C-terminus of circularly permuted GFP (cpGFP), respectively (Fig. 2). Since both N-terminus and C-terminus of cpGFP is located in the vicinity of chromophore, the antigen binding to VH and VL region causes a change in the environment surrounding the chromophore, leading to fluorescence intensity change. Our first prototype utilized the antibody against short peptide from Bone Gla Protein (BGPC7; RRFYGPV), which has been shown to bind its antigen in living cells. When BGPC7 peptide was added, the prototype BGP sensor showed a slight fluorescence intensity change. To expand the dynamic range, we altered the length of amino acid linkers and mutated each amino acid in the linkers between antibody and fluorescent protein. After an extensive screening, we finally developed a biosensor with 4-fold fluorescence change with addition of the antigen, and named the biosensor as "Flashbody" after the chemically-fluorescence labeled antibody sensor "Quenchbody" previously developed in our laboratoryref2.
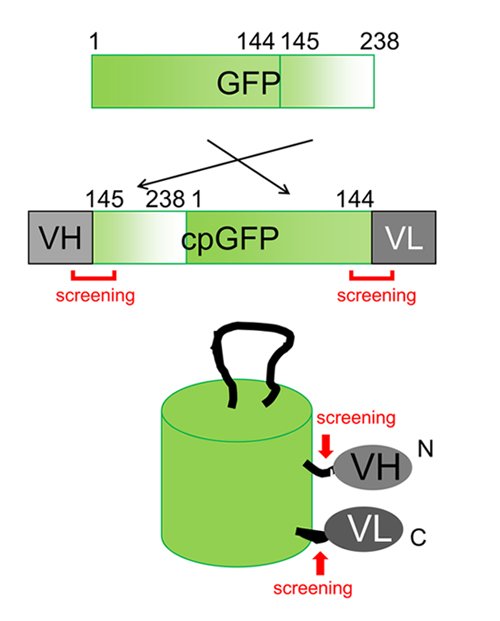 Figure 2
Figure 2
Construction of Flashbody by cpGFP and heavy/light chain of variable region of antibody.
Spectrophotometric characterization of BGP Flashbody protein revealed that it emits green fluorescence with a peak at 513 nm, similar to the emission peak of cpGFP, and fluorescence intensity was increased by BGPC7 peptide in a dose dependent manner (Fig. 3). Moreover, different peptides such as Myc peptide did not induce fluorescence intensity change. We then examined the applicability of Flashbody in the living cells (Fig. 4). After BGP Flashbody was expressed in the cytoplasm or nucleus in HeLa cells, the BGPC7 peptide fused with cell penetrating peptide (CPP) from flock house virus (FHV) was added extracellularly. As the peptide translocated through the plasma membrane and nuclear membrane, the fluorescence intensity of Flashbody was increased in both cytoplasm and nucleus. Interestingly, the fluorescence intensity increase at the plasma membrane was spatially confined and rapidly emerged as bright spots, while fluorescence intensity in the nucleus was gradually increased. Flashbody was able to visualize that the mechanism of CPP penetration across the plasma membrane is different from the nuclear membrane.
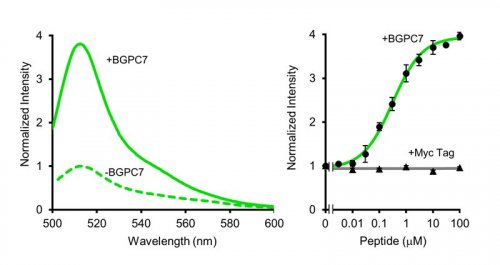 Figure 3ref1
Figure 3ref1
Spectral property of Flashbody (left) and a dose-response relationship (right). (©American Chemical Society)
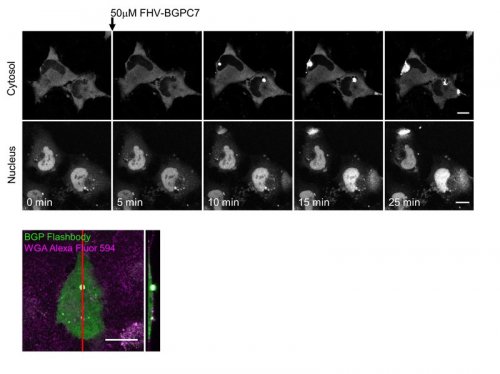 Figure 4ref1
Figure 4ref1
Sequential images of visualization of translocation of FHV-antigen by Flashbody in living cells (top). Z-projection image of the entry point of antigen (bottom). (©American Chemical Society)
Flashbody is expected to dramatically improve the diversity of molecular recognition for fluorescent protein sensors because this domain is exchangeable to other antibodies which generally share a similar structure. Since Flashbody is genetically-encoded, it can be used not only for intracellular imaging for specific organelles in living cells but also for in vivo imaging in transgenic animals and plants. Besides the fluorescence imaging under optical microscope, purified Flashbody is applicable to clinical examinations, environmental survey, food inspection etc. In the future, we will develop various Flashbody with myriad antibodies and fluorescent proteins of different colors, and simultaneously visualize and detect diverse molecules with multiple colors in the living cells or clinical samples.
References
[1] D. Wongso, J. Dong, H. Ueda, T. Kitaguchi, Anal. Chem., 2017, 89, 6719-6725.
[2] R. Abe, H. Ohashi, I. Iijima, M. Ihara, H. Takagi, T. Hohsaka, H. Ueda. J. Am. Chem. Soc. 2011, 133, 17386-17394.



