Latest Research
- 2018.03.01
Elucidation of zeolite acidity and proton hopping mechanism at high temperature using infrared spectroscopy
Zeolites are formed by (SiO4)4- and (AlO4)5- units, and they are crystalline microporous materials with 3-dimentional structures. 235 types of framework structures have been reported so far. Thus, the characteristic molecular sieve function is appeared depending on the porous structure, which shows the specific catalytic selectivity for several reactions. Among them, the application of zeolites as solid acid catalysts has been extensively investigated by many researchers1). Fig. 1 shows framework structure of MFI type zeolite and schematic diagram of Brønsted acid sites in zeolite framework. The bridging OH groups between Si and Al atoms are Brønsted acid sites, which can be observed by infrared (IR) spectroscopy as O-H stretching vibrations.
Fig. 1. Framework structure of MFI type zeolite and schematic diagram of acid sites.
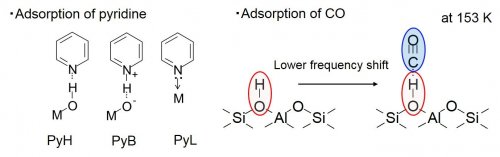
The basic probes such as pyridine and CO are widely used for the conventional characterization method of zeolite acidity using IR 2) (Fig. 2). However, one of the disadvantages lies on the gap of temperatures between characterization and catalytic reactions3).
Fig. 2. Conventional characterization methods for zeolites acidity.
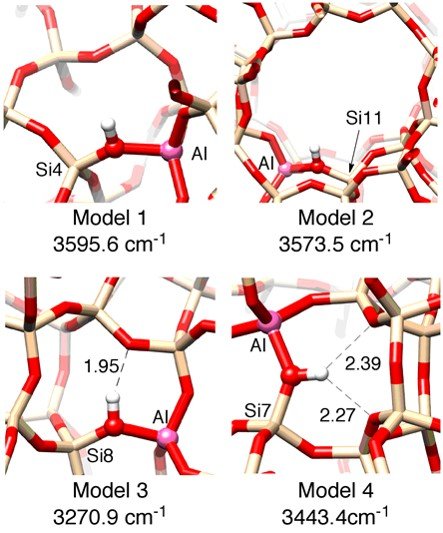
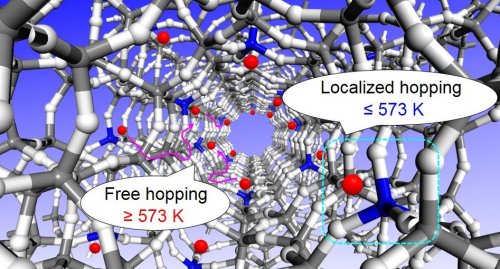
In the present study, the dissociation enthalpy of acidic OH groups was estimated by in-situ FT-IR system for characterizing the zeolites acidity at high temperatures, where different zeolite topologies were compared4). As a result, the dissociation enthalpy was affected by the zeolite topology despite the same chemical composition as bridging OH groups between Si and Al atoms, and different values of dissociation enthalpy were obtained in different temperature area. This tendency was obtained with all zeolite topology in the investigated samples. These results were supported by the results of density functional theory (DFT) calculation, where the different IR bands and energies were estimated on different oxygen atoms in the same (AlO4)5- units (Fig. 3). Therefore, the acidity of zeolites at the high temperatures is strongly related to the proton-hopping mechanism, which were proposed the two types mechanisms as shown in Fig. 4. The elucidation of the zeolite acidity at high temperatures is, thus, very important for the design of catalysts.
Fig. 3. Four models of O−H stretching frequencies on several oxygen atoms.
Fig. 4. Proton hopping mechanisms of zeolite at high temperatures.
This research is collaborated research with Dr. Kazuki Doitomi and Prof. Hajime Hirao (City University of Hong Kong).
1) A. Corma, Chem. Rev. 1995, 95, 559−614.
2) G. Busca, Microporous Mesoporous Mater. 2017, 254, 3−16.
3) K. Chakarova, K. Hadjiivanov, J. Phys. Chem. C 2011, 115, 4806−4817.
4) R. Osuga, T. Yokoi, K. Doitomi, H. Hirao, J. N. Kondo, J. Phys. Chem. C 2017, 121, 25411-25420.