Latest Research
- 2018.05.01
- Tanaka-Yoshida Group
Target of rapamycin: linking chloroplast and cytosolic ribosome biogenesis in plants
Chloroplasts are crucial specialized structures within a cell that generate energy and produce storage- and cell metabolism-related molecules that underpin cell growth. The reactions that take place in chloroplasts require the synthesis of proteins by the chloroplast ribosome; however, how this is regulated in plant cells is still largely unknown. The conversion of ribosomal DNA to ribosomal RNA (rRNA), i.e., transcription, is thought to be a rate-limiting step of ribosome biogenesis, and hence it is crucial to clearly understand rRNA transcription [1].
Previous studies have revealed that inhibiting TOR (target of rapamycin), a highly conserved protein kinase [2], represses nuclear rRNA transcription in many eukaryotes [3], and hence we hypothesized that TOR also regulates rRNA transcription in chloroplasts and mitochondria. We found that TOR modulates the transcription of chloroplast rRNA, as well as that of nuclear and mitochondrial rRNA, in a model unicellular red alga, Cyanidioschyzon merolae (Fig. 1). The result indicates that TOR is involved in a mechanism that regulates biogenesis in all three ribosomes (Fig. 2).
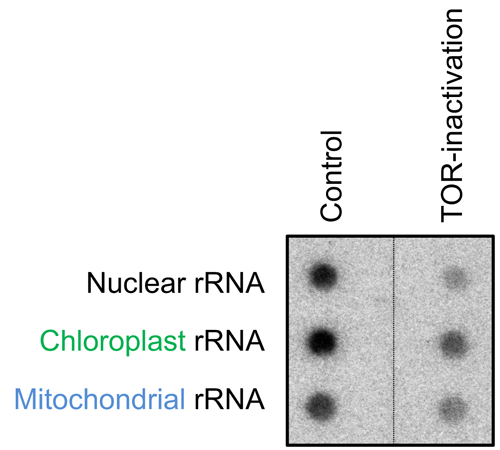 Figure 1.
Figure 1.
TOR-inactivation causes inhibition of rRNA transcription in the nucleus, chloroplast and mitochondrion.
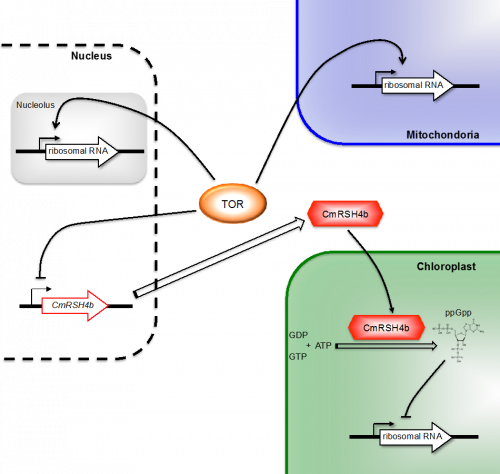 Figure 2.
Figure 2.
A linked regulatory mechanism for biogenesis of the three ribosomes by TOR.
But how does TOR regulate chloroplast rRNA transcription in a cell? We tried to answer this question by analyzing the effects of TOR inhibition. We found that this inhibition increased the expression of a nuclear-encoded chloroplast gene, CmRSH4b, which encodes a homolog of the signal molecule guanosine 3′-diphosphate 5′-diphosphate (ppGpp) synthetases that modulates rRNA synthesis in bacteria. Further genetic and biochemical analyses demonstrated that the synthesis of CmRSH4b-dependent ppGpp in chloroplasts is an important regulator of chloroplast rRNA transcription (Fig. 2)[4].
From an evolutionary standpoint, chloroplasts are considered to have originated from an endosymbiotic event of a cyanobacteria-like bacterium with a non-photosynthetic host eukaryotic cell. TOR is a master regulator that integrates nutrient and energy status to control cell proliferation and growth in eukaryotic cells, while the ppGpp-centered regulatory system plays similar physiological roles in bacterial cells [5]. We suggest that these eukaryotic and bacterial systems of independent origins were connected in a primitive alga, which may indicate that coexistence of both systems was essential and thereafter conserved during algal and plant evolution (Fig. 3).
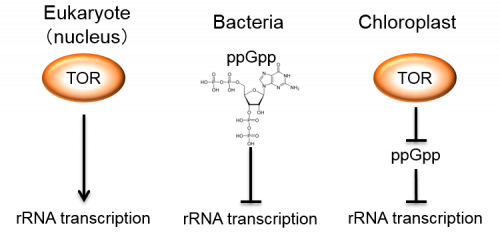 Figure 3.
Figure 3.
Eukaryotic and bacterial growth regulation systems of independent origins are connected to control chloroplast rRNA transcription.
The results of this study lay the foundation for future research in chloroplast ribosome biogenesis. Understanding the coordination between the three interconnected ribosome systems will allow for further insights into the regulation of growth of algal and complex plant cells.
[1] Hannan K.M., Hannan R.D. and Rothblum L.I. (1998) Transcription by RNA polymerase I. Front Biosci. 3, d376-d398.
[2] Laplante M. and Sabatini D.M. (2012) mTOR signaling in growth control and disease. Cell 149, 274-293.
[3] Mayer C., Zhao J., Yuan X. and Grummt I. (2004) mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev. 18, 423-434.
[4] Imamura S., Nomura Y., Takemura T., Pancha I., Taki K., Toguchi K., Tozawa Y. and Tanaka K. (2018) Plant J. 94, 327-339.
[5] Atkinson, G.C., Tenson, T. and Hauryliuk, V. (2011) The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS One 6, e23479.



