Latest Research
- 2018.06.01
- Nishiyama-Miura Group
Construction of ethylenediamine-based polyzwitterion for effective accumulation of the coated nanomaterials in tumor
Surface of biomaterials determines their interaction with biological substances in a body. In particular, the nanomaterials loading anticancer drugs and imaging reagents should reach the tumor site by avoiding the undesired interaction with blood components and/or normal tissues1. To this end, the nanomaterials has been often coated with antifouling polymers, such as poly(ethylene glycol) (PEG)2. In this regard, polyzwitterion, which comprises betaine structures in the side chains, has also attracted increasing attention as the antifouling polymers3.
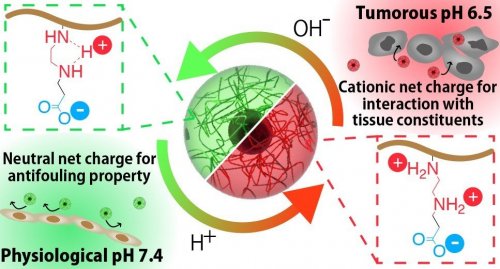
In general, betaine structure has two vicinal opposite charges, and it exhibits antifouling property when the net charge is neutral. Therefore, polyzwitterion has been designed to be neutral at physiological pH 7.4 for coating the biomaterials. In this regard, tumorous acidic pH has triggered our interest in developing a smart polyzwitterion that shows switchable properties in response to tumorous condition; tumorous pH is ~6.5 due to the dominant anaerobic respiration and augmented excretion of acidic metabolites (i.e., Warburg effect)4. Toward the development of the smart polyzwitterion, ethylenediamine moiety was employed in the present study. Ethylenediamine structure is mono-protonated at physiological pH, but di-protonation is initiated at acidic pH5. Thus, polyzwitterion comprising ethylenediamine moiety as a cationic group would potentially exert antifouling property during blood circulation and against normal tissues, but likely to accumulate in tumorous tissues through the interaction with tissue constituents (Figure 1)6.
(Figure 1. Illustration of the nanomaterials with a coating polymer developed in the present study)
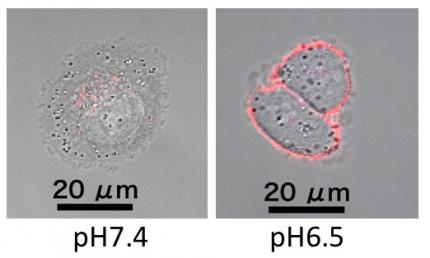
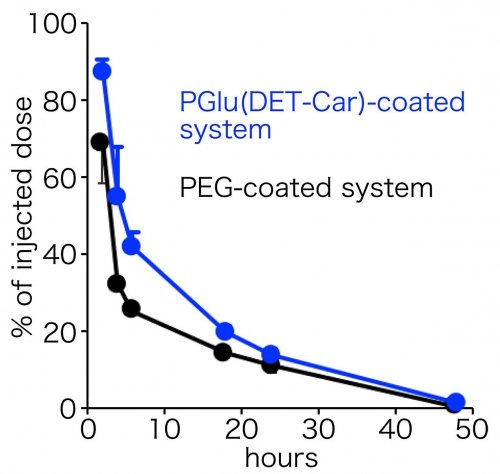
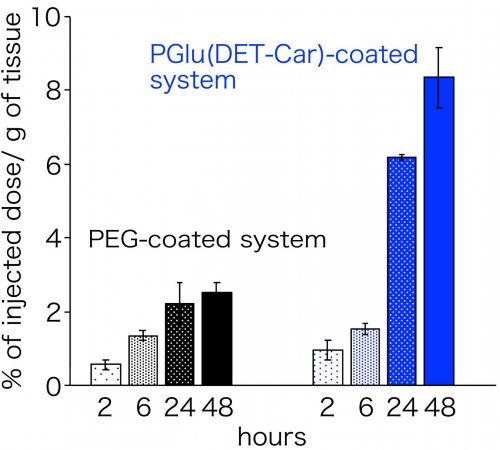
The smart polyzwitterion was synthesized (termed as PGlu(DET-Car)), and its pH-responsiveness was investigated for the interaction with cultured cells (Figure 2). As a result, PGlu(DET-Car) did not interact with the cells at pH 7.4, but the appreciable residence on the cells was observed at pH 6.5. This acidic pH-responsive interaction with cells indicates that neutral net charge of PGlu(DET-Car) was shifted to be cationic at tumorous pH, leading to the interaction with anionic cellular membrane. Next, nanomaterials (quantum dots in this study) was coated with PGlu(DET-Car), followed by administration for mice bearing subcutaneous tumor. Herein, PEG system for conventional antifouling control and cationic system for interactive control were also prepared. The nanomaterials coated with PGlu(DET-Car) showed similar blood circulation profile and distribution for normal tissues, relative to PEG system (Figure 3). Thus, coating with PGlu(DET-Car) achieved comparable antifouling property for normal tissues to PEG. The short circulation in blood and the accumulation in lung for cationic system, where cationic nanomaterials often undergoes aggregation with blood components followed by the entrapment by lung capillary, also confirmed antifouling property of PGlu(DET-Car). Importantly, PGlu(DET-Car) system resulted in ~3 times higher accumulation of the coated nanomaterials compared to PEG system (Figure 4). Considering that the two systems showed similar performance for blood circulation and distribution in normal tissues, the difference should be attributed to the events in tumor tissues; in tumorous tissues, PGlu(DET-Car) changes its property to be cationic leading to interaction with tumorous constituents, while it is antifouling during blood circulation.
(Figure 2. pH-responsive interaction of PGlu(DET-Car) with cultured cells)
(Figure 3. Blood circulation profile of the polymer-coated nanomaterials)
(Figure 4. Tumor accumulation property of the polymer-coated naomaterials)
In the present study, we successfully demonstrated that tuning of pH-responsiveness of betaine structure leads to the development of smart polyzwitterion that enables effective accumulation of the coated nanomaterials in tumor tissues. The system is now in the study for further investigation of ionizable moieties and medicinal application by loading of drugs.
References
[1] G. Morgese, B. Shirmardi Shaghasemi, V. Causin, M. Zenobi-Wong, S. N. Ramakrishna, E. Reimhult, E. M. Benetti, Angew. Chem. Int. Ed. 2017, 56, 4507-4511.
[2] A. L. Klibanov, K. Maruyama, V. P. Torchilin, L. Huang, FEBS Lett. 1990, 268, 235-237.
[3] A. K. Nowinski, F. Sun, A. D. White, A. J. Keefe, S. Jiang, J. Am. Chem. Soc. 2012, 134, 6000-6005.
[4] R. K. Jain, T. Stylianopoulos, Nat. Rev. Clin. Oncol. 2010, 7, 653-664.
[5] K. Miyata, M. Oba, M. Nakanishi, S. Fukushima, Y. Yamasaki, H. Koyama, N. Nishiyama, K. Kataoka, J. Am. Chem. Soc. 2008, 130, 16287-16294.
[6] A. H. Ranneh, H. Takemoto, S. Sakuma, A. Awaad, T. Nomoto, Y. Mo-chida, M. Matsui, K. Tomoda, M. Naito, N. Nishiyama, Angew. Chem. Int. Ed. 2018, 57, 5057-5061.