Latest Research
- 2018.07.09
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
On the vibrational resonance state of the benzene dimer excimer studied by infrared spectroscopy
Consideration of baking condition of a double-decker waffle
Benzene, which has a regular hexagonal shape like a turtle shell pattern, is one of the most famous and important molecules. Understanding of benzene provides fundamental information about constitution of our world that consists of assembly of many molecules. Although the carbon atoms constructing the frame are arranged in a regular hexagon, it looks a roll with some angular parts in practice, because the frame is wrapped by a cloud of electrons. Or a waffle would be more similar as it has some bumps on the surface as shown in the picture. Let me introduce how to make a double-decker waffle this time.
The waffles will peel off immediately if you just stack waffles. In order to pile up waffles, you can think of inserting jam or pinching them from both sides (chemists do something similar). Besides that, there is a peculiar way in the very very small world of molecules.
That is, to change the baking condition of one of the "same" waffles, making one of them scorched (called "electronic excitation") and then overlapping them. Perhaps you think what is different from simply overlapping two waffles. But here is the world of molecules. There is a principle in quantum mechanics, which govern not only the molecular world but also our world, that the "same" things cannot be distinguished. Even if it is scorched a little, the "same" waffle is still the "same". We will not know which waffle was put on above or below, "scorched-regular" or "regular-scorched". This way of piling is called "Excimer".
In this situation, the scorch is entangled between the two waffles. They stick each other and will not come off (for a while). Such excimers are interested in not only sticking two molecules but also variety of applications, e.g. fluorescent probes due to its funny emission, and damages on skin by sunlight as excimers may happens when DNA is irradiated by UV light.[1,2]
Then, what has become of the relation of upper and lower of the waffles at this time?
1. It is a parallel world, only one combination exists in each world.
2. One time the upper waffle is scorched, while the lower is in
another time
3. The scorch is mixed up in both, and the waffles are done to a
beautiful brown.
The answer is 3., both waffles are baked to a beautiful brown.
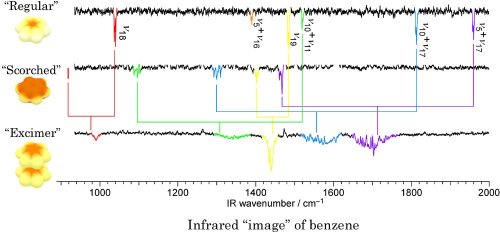
This fact is confirmed by watching "regular", "scorched", and "excimer" waffles by a tool like infrared night vision scope (called IR spectroscopy). This "infrared scope" burns the waffles a little bit using far infrared light. Since patterns of burning by far infrared light are different depending on the baking conditions of the waffles, the waffles can be looked different each other. The infrared scope images (called "spectra") are shown in below. The top most shows that of the "regular" waffle, the middle "scorched" one, and the bottom most corresponds to the double-decker "excimer" waffle. It has already well known how the infrared scope image changes depending on the baking condition of a waffle, because the waffle is very important molecule in chemistry. Indices and numbers written in a side of each dip tell us the information. The infrared image of the double-decker "excimer" waffle was observed by us for the first time.[3] Dips connected by lines with the same color represent a feature (called "vibrational mode") of the waffle in each baking condition. You can notice that positions of dips change in each baking condition.
In the double-decker "excimer" waffle, the dip of each feature appears in nearly the middle of corresponding positions of "regular" and "scorched" ones. The reason that the width of dips is wider is because the double-decker waffle is heated and is hot a bit. It is, however, not easy to know baking condition of waffles in the excimer from the infrared scope image. Consideration on the basis of quantum mechanics is required. Here a detailed proof is omitted, we have proved the relation between the infrared image and baking condition in the double-decker "excimer" waffle in the paper [3]. From the results, we concluded that the dips appear in the middle of corresponding dip positions of "regular" and "scorched" waffles if the scorch is entangled in both the waffles in the double-decker excimer, and both the waffles are baked to a good brown color. The "vibrational resonance" in the title of the document means such a situation.
Incidentally, the waffle made of benzene expands when it is burnt. In the double-decker excimer waffle, the size of waffles is also in between those of "regular" and "scorched" by entangling the scorch half-and-half. Degrees of scorch and expansion both automatically become half-and-half by merely piling waffles with different baking conditions. This is a wonder of quantum mechanics. Please visit the Laboratory for Chemistry and Life Science. There should be plenty of other dishes, drinks, and more. Although I do not know whether you can easily eat them, because molecules are full of whims and fancies.
[1] C. E. Crespo-Hernández, B. Cohen, P. M. Hare and B. Kohler,
Chem. Rev. 104, 1977-2019 (2004).
[2] M. D. Ediger, Annu. Rev. Phys. Chem. 42, 225-250 (1991).
[3] M. Miyazaki and M. Fujii, Phys. Chem. Chem. Phys. 19,
22759-22776 (2017).