Latest Research
- 2018.08.08
- Nakamura-Okada Group
Rapid and Mild Synthesis of Amino Acid N-Carboxy Anhydrides Using Basic-to-Acidic Flash Switching in a Micro-flow Reactor
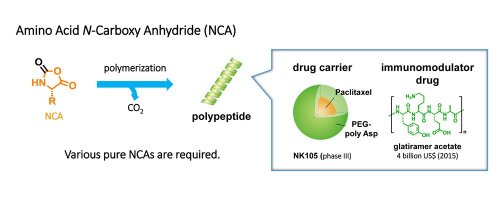
Polymerization of N-carboxy anhydrides (NCAs) is the primary process used to prepare polypeptides. The synthesis of various pure NCAs is a key to the efficient synthesis of polypeptides (Figure 1).
The only practical method that can be used to synthesize NCAs requires harsh acidic conditions that make acid-labile substrates unusable and results in an undesired ring opening of NCAs. The Fuchs-Farthing method was first reported in 1922 and remains the only practical method for NCA synthesis.1) In this process, amino acids are coupled with phosgene to afford the intended NCAs with the generation of HCl. This one-step process only emits CO2 and HCl; the NCAs that are produced are relatively stable under acidic conditions, and there is no undesired polymerization of NCAs. However this process requires harsh conditions (ca. 40-50 oC, 2-5 h, pH <1) and prohibit the use of amino acids with acid-labile side chains. Moreover, the extended heating of NCAs with HCl sometimes causes an undesired ring opening of NCAs to generate acid chloride (Scheme 1, upper scheme), and this leads to a decrease in purity. If NCA formation is carried out under basic conditions, amino acids will rapidly attack phosgene at ambient temperatures (Scheme 1, lower scheme). However, deprotonation of the NH groups in the produced NCAs causes their undesired polymerization. Therefore, NCA synthesis under basic conditions has never been reported.
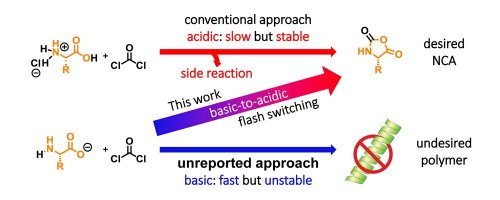 Scheme 1. Traditional synthesis of NCA (upper scheme), unreported synthesis of NCA (lower scheme), and developed synthesis of NCA (from lower left to upper right).
Scheme 1. Traditional synthesis of NCA (upper scheme), unreported synthesis of NCA (lower scheme), and developed synthesis of NCA (from lower left to upper right).
"The desired reaction proceeds smoothly under basic conditions, however, basic conditions induce undesired polymerization of the product."
How can we solve this general problem in organic synthesis?
Our solution is
"Basic-to-acidic flash switching in a micro-flow reactor in 0.1 sec."
(Scheme 1, from lower left to upper right)
We anticipated that the desired NCA formation rapidly occurs under basic conditions and the desired NCA can be obtained without undesired polymerization after basic-to acidic switching in 0.1 sec. In order to achieve the flash switching, aqueous solution of an amino acid and organic solution of triphosgene (= phosgene equivalent) have to be mixed in 0.1 sec. This rapid mixing cannot be achieved under traditional batch conditions whereas, the micro-flow conditions enable the rapid mixing less than 0.1 sec.
We achieved the flash switching of pH using HCl generated from excessively added triphosgene and H2O solvent in a micro-flow reactor (Figure 2). In order to avoid acidic decomposition of acid-labile NCAs, flash dilution with EtOAc was performed and achieved the synthesis of various NCAs from all the proteinogenic amino acids and several acid-labile nonproteinogenic amino acids.2)
Although NCAs are important materials, strict control of temperature for storage and transportation is required due to their instability that limits their utility. Because micro-flow synthesis is readily scalable and space-saving, the developed approach should pave the way for on-demand, on-site synthesis of NCAs with expanded utility.
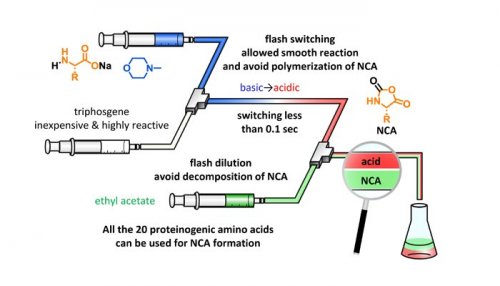 Figure 2. Micro-flow synthesis of NCA.
Figure 2. Micro-flow synthesis of NCA.
1) a) F. Fuchs, Ber. Dtsch. Chem. Ges. 1922, 55, 2943-2943;
b) A. C. Farthing, J. Chem. Soc. 1950, 3213-3217.
2) Y. Otake, H. Nakamura, S. Fuse Angew. Chem. Int. Ed.
accepted for publication