Latest Research
- 2018.12.27
Progress in Radical Fluoroalkylation by Organic Photoredox Catalysis
Fluoromethyl groups such as the trifluoromethyl (CF3) and difluoromethyl (CF2H) groups are useful structural motifs, especially in the fields of pharmaceuticals and agrochemicals, because they can improve ADME (absorption, distribution, metabolism, and excretion of drug).1-3 Therefore, development of simple methodologies for efficient and selective incorporation of the fluoromethyl groups into various organic skeletons is highly demanded. Use of reactive fluoroalkyl radicals is a promising strategy.4 Recently, our group has developed radical fluoromethylation by action of ruthenium- and iridium-based photoredox catalysts.5 Herein recent progress on radical fluoroalkylation by organic photoredox catalysis in our group will be described.6
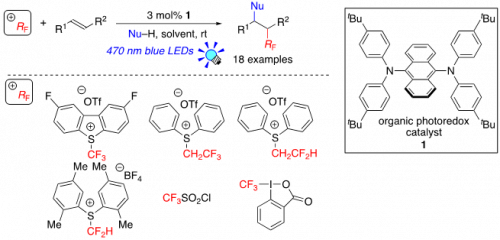
We paid our attention to 9,10-bis(di(p-tert-butylphenyl)amino)anthracene (1), which is a latent organic photoredox catalyst with high reducing power. We expected that the photoexcited 1 undergoes SET to electron-accepting fluoroalkylating reagents (+RF) to generate fluoroalkyl radicals (·RF) such as tri- and di-fluoroethyl and tri- and di-fluoromethyl radicals. In fact, 1 showed excellent photocatalytic activities with respect to various fluoroalkylation of alkenes (Figure 1).
Fig.1
Radical fluoroalkylation of alkenes by organic photoredox catalysis
From the viewpoint of elemental strategy initiative and green chemistry, the present noble metal-free organic photocatalytic system provides a pivotal technology to replace ruthenium- and iridium-based metal photocatalysis. The new photocatalytic reaction system is expected to see extensive use in the field of medicinal/agrochemical product development in the future.
References
(1) I. Ojima, Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: Chichester, 2009.
(2) P. Kirsch, Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, 2013.
(3) N. A. Meanwell, J. Med. Chem., 2011, 54, 2529-2591.
(4) A. Studer, Angew. Chem. Int. Ed., 2012, 51, 8950-8958.
(5) T. Koike, M. Akita, Acc. Chem. Res., 2016, 49, 1937-1945.
(6) N. Noto, Y. Tanaka, T. Koike, M. Akita, ACS Catal., 2018, 8, 9408-9419.