Latest Research
- 2019.02.20
- Fukushima-Shoji Group
Only two small triptycene units dramatically change the structural and physical properties of an amorphous polymer
Thin films are an important form of materials for many practical applications. In the design of organic thin films, precise control of the arrangement and orientation of the constituent molecules beyond micrometer scales to obtain a large-area uniform film is a big challenge. We have recently reported that 1,8,13-substututed triptycene derivatives can self-assemble into a "2D hexagonal array + 1D lamellar" structure through nested packing of the triple blades of the triptycene framework to afford a perfectly oriented macroscopic thin film on various substrates1,2. This type of triptycene-based 2D materials were found to be useful as a surface modifier for organic polymer substrates3. Furthermore, the "2D + 1D" assembly motif of the tripodal triptycene serves as a robust supramolecular scaffold, which enables the controlled assembly of functional molecular units. For example, we successfully demonstrated that a tripodal triptycene with a C60 unit at the bridgehead position gives rise to an oriented thin film, where the C60 units are densely clustered two-dimensionally4.
Figure 1. Schematic illustration of the "2D + 1D" assembly structure of tripodal triptycenes on the surface of a solid substrate.
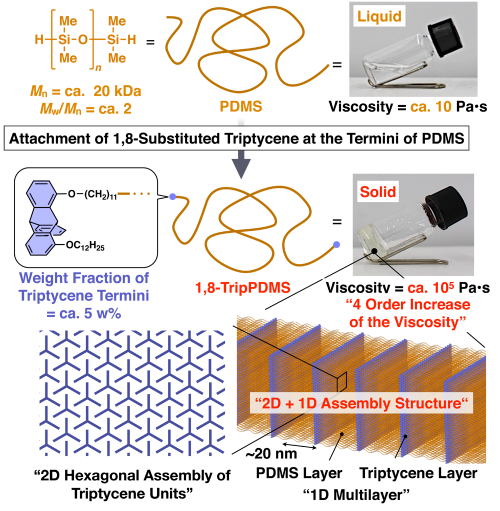
Meanwhile, tailoring a periodically ordered structure with polymer assemblies is also an important subject to develop various advanced soft materials that allow e.g., nanopatterning and directional (ion-, charge-, and heat-) transporting, as well as optical and mechanical applications. We anticipated that the robust self-assembling ability of the triptycene would result in structural ordering of polymer systems when suitably functionalized. With this in mind, we synthesized a telechelic polydimethylsiloxane (PDMS, Mn = ca. 20 kDa, Mw/Mn = ca. 2) with 1,8-substitued triptycene moieties at the termini (Figure 2). Notably, X-ray scattering experiments of the resulting PDMS revealed the formation of a "2D + 1D" structure, where layers of PDMS domains, formed between 2D assemblies of the triptycene termini, stack into a 1D multilayer structure with a layer spacing of 20 nm. Due to this particular structure, the complex viscosity of the telechelic PDMS was dramatically enhanced, providing a value four orders of magnitude greater than that of original PDMS. The present finding will update the general notion that terminal functionalization is not effective to achieve the controlled assembly of polymers into a highly ordered structure. The tripodal triptycene motif may provide a powerful tool for organizing polymers and in turn for reinforcing structural and physical properties. Studies along these lines are in progress by our group.
Figure 2. Schematic illustration of the structuring behavior of 1,8-TripPDMS that leads to dramatic change in the physical properties of the original PDMS.
References
- 1) As a review: F. Ishiwari, Y. Shoji, T. Fukushima, Chem. Sci. 2018, 9, 2028-2041.(Open Access)
- 2) N. Seiki, Y. Shoji, T. Kajitani, F. Ishiwari, A. Kosaka, T. Hikima, M. Takata, T. Someya, T. Fukushima, Science 2015, 348, 1122-1126.
- 3) T. Yokota, T. Kajitani, R. Shidachi, T. Tokuhara, M. Kaltenbrunner, Y. Shoji, F. Ishiwari, T. Sekitani, T. Fukushima, T. Someya, Nature Nanotech. 2018, 13, 139-144.
- 4) F. K.-C. Leung, F. Ishiwari, T. Kajitani, Y. Shoji, T. Hikima, M. Takata, A. Saeki, S. Seki, Y. M. A. Yamada, T. Fukushima, J. Am. Chem. Soc. 2016, 138, 11727-11733.
- 5) F. Ishiwari, G. Okabe, H. Ogiwara, T. Kajitani, M. Tokita, M. Takata, T. Fukushima, J. Am. Chem. Soc. 2018, 140, 13497-13502.