Latest Research
- 2019.06.11
- Osakada Group
Multinuclear transition metal complexes having bridging Si or Ge ligands
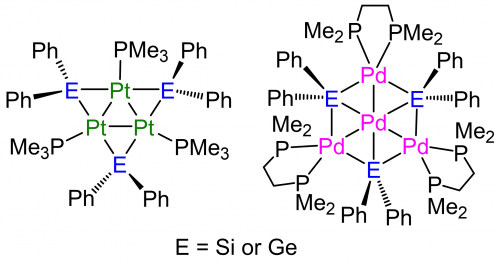
Our research group has reported new multinuclear transition metal complexes such as trinuclear platinum complexes having bridging silylene1 or germylene2 ligands, and tetranuclear palladium complex having similar Si- and Ge- ligands for these two decades (Figure 1).3,4 These complexes show various chemical properties that differ from already reported multinuclear complexes stabilized by bridging electron-withdrawing ligands, such as CO and CNR. Several substrates react with the complexes keeping the multinuclear structure stabilized by metal-metal bonds and bridging electron-donating ligands.5
Figure 1. Structures of multinuclear transition complex having bridging silylene or germylene ligands.
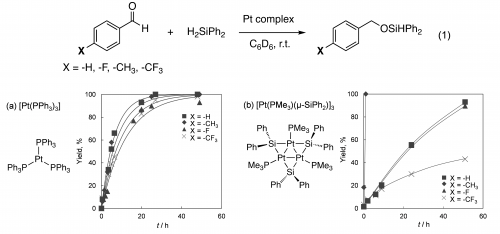
Complexes of various late transition metals such as Rh, Cu, Fe, and Ni, were reported to catalyze hydrosilylation of carbonyl compounds such as aldehydes and ketones. There have been only a limited number of reports on the hydrosilylation of carbonyl compounds using Pt catalyst, although molecular Pt complexes and Pt salts are known as the efficient catalyst for hydrosilylation of olefins. We found the trinuclear Pt complex (1) catalyzed the hydrosilylation of aryl aldehydes, which afforded aryl silyl ether in high selectivity (Eq. 1).6 After completion of the reaction, the 31P NMR signals of Pt3 complex (1) did not change, suggesting that the reaction was catalyzed by the trinuclear Pt complex rather than by its decomposition product. Reaction of aromatic aldehydes, such as PhCHO, 4-FC6H4CHO, 4-MeC6H4CHO and 4-CF3C6H4CHO with H2SiPh2 in the presence of [Pt(PPh3)3] catalyst proceeds smoothly at room temperature with similar reaction rates, while large dependence of the reaction rate was observed by Pt3 complex (1) (Figure 2).
Figure 2. Formation of Si-O bond catalyzed by platinum complexes.
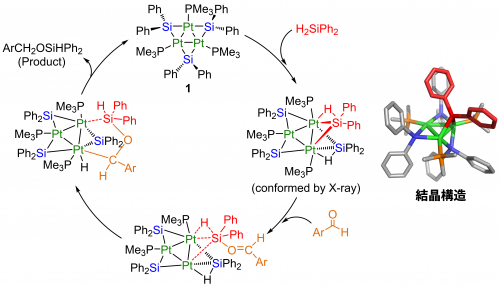
Based on the substituent effects, isolation of intermediate, and comparison with reported examples, we concluded that these reactions proceeded via different catalytic pathways. Pt3 complex (1) catalysis probably involves O-Si bond formation at the initial stage of the reaction, followed by C-H bond formation (Figure 3), and the [Pt(PPh3)3] catalysis is considered to proceed via insertion of C=O bond into the Pt-H bond.
Figure 3. Proposed catalytic mechanism
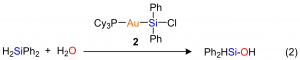
Currently, we are studying transition metal complexes with Au(I), which have similar electron configuration with Pd(0) and Pt(0). We have prepared a new gold-silyl complex (2) having Au-Si bond. This complex can catalyze the hydrogenation of H2SiPh2, which afforded corresponding silyl alcohol, Ph2HSiOH, in high yield and selectively (Eq. 2)8. Silyl alcohol delivers silicone, which is an industrially important material. Typical procedures, however, require a strong reducing reagent and produces HCl as a toxic by-product. In this study, we obtained silyl alcohol in high selectivity without toxic by-product by the hydrogenation of H2SiPh2 in the presence of gold-silyl complex (2).
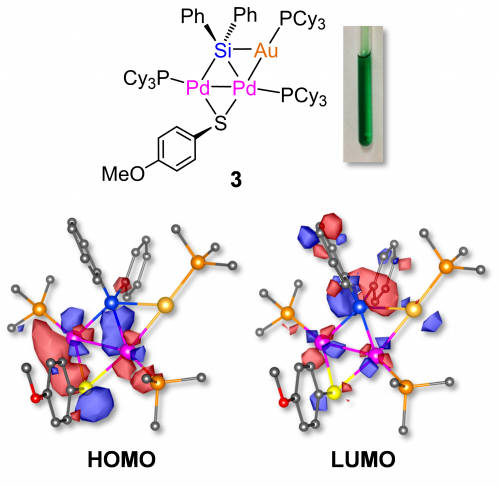
We have prepared a new AuPd2 trinuclear complex (3), which consist of one Au and two Pd cores connected by silylene ligand9. The AuPd2 trinuclear complex exhibits deep green color (λmax = 631 nm in toluene), which caused by intramolecular charge transfer absorption from Pd2S group (HOMO) to AuSi group (LUMO) based on the DFT calculations (Figure 4).
Figure 4. Molecular structure and orbitals of trinuclear AuPd2 complex (3).
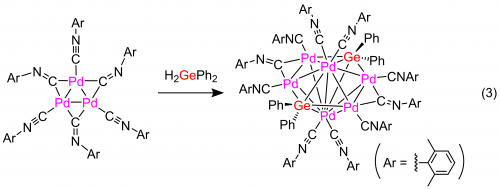
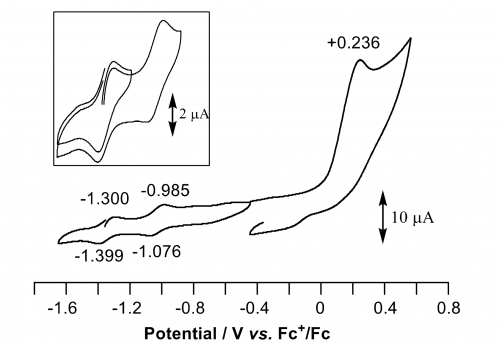
Recently, we succeeded in selective synthesis of a hexanuclear Pd complex with three-dimensional geometry and its redox behavior.10 The reaction of a planar tripalladium(0) complex with H2GePh2 to afford complex 4 having a new structure with a hexagonal bipyramidal core. Single-crystal X-ray crystallography analysis revealed that complex 4 consists of four Pd and two Ge atoms forming a three-dimensional hexagonal conformation. The two pairs of Pd centers are bridged by the isonitrile ligands. Electrochemical oxidation occurs via two successive one-electron processes with high reversibility, which indicates that the Pd6Ge2 core is stable both in the reduced and in the oxidized state (Figure 5).
Figure 5. Cyclic voltammogram of Pd6Ge2 complex
- 1) K. Osakada, M. Tanabe, T. Tanase, Angew. Chem., Int. Ed. 2000, 39, 4053-4055.
- 2) M. Tanabe, K. Tanaka, S. Omine, K. Osakada, Chem. Commun. 2014, 50, 6839-6842.
- 3) T. Yamada, A. Mawatari, M. Tanabe, K. Osakada, T. Tanase, Angew. Chem., Int. Ed. 2009, 48, 568-571.
- 4) M. Tanabe, N. Ishikawa, M. Chiba, T. Ide, K. Osakada, T. Tanase, J. Am. Chem. Soc., 2011, 133, 18598-18601.
- 5) M. Tanabe, R. Yumoto, T. Yamada, T. Fukuta, T. Hoshino, K. Osakada, T. Tanase, Chem. Eur. J. 2017, 23, 1386-1392.
- 6) Makoto Tanabe, Megumi Kamono, Kimiya Tanaka, Kohtaro Osakada, Organometallics 2017, 36, 1929-1935.
- 7) Y. Tsuchido, R. Abe, M. Kamono, K. Tanaka, M. Tanabe, K. Osakada, Bull. Chem. Soc. Jpn. 2018, 91, 858-864.
- 8) 神田 篤志,丹羽 孝明,土戸 良高,小坂田 耕太郎, 第8回CSJ化学フェスタ ポスター発表(P6-063), ポスター賞受賞
- 9) 大熊 一輝・神田 篤志・土戸 良高・小坂田 耕太郎, 第99回日本化学会春季年会 口頭発表(1D1-32)
- 10) T. Koizumi, K. Tanaka, Y. Tsuchido, M. Tanabe, T. Ide, K. Osakada, Dalton Trans. 2019, 48, 7541-7545.