Latest Research
- 2019.07.01
- Yamaguchi-Kuroki Group
Polyelectrolyte membrane for solid-alkaline fuel cell: Design and synthesis of highly durable anion conducting polymer
Due to the recent development and prevailing of power supplies like solar cell, low-cost renewable energy will be easily obtained soon. Fuel cell is a very important device for converting unstable renewable energy supply to chemical energy and producing electricity whenever necessary.
Polymer electrolyte fuel cell (PEFC) using proton exchange membrane (PEM) is commercially in use because this device can be operated under low temperature and shows relatively high energy conversion efficiency (Fig 1 left).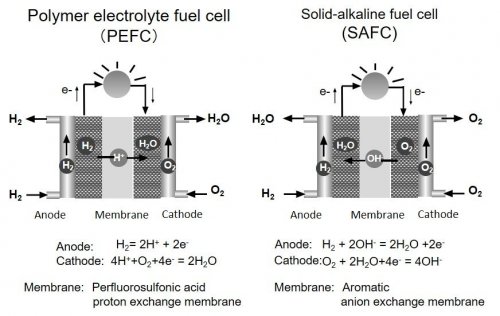
Fig 1.Structure of polymer electrolyte fuel cell (PEFC) and solid-alkaline fuel cell (SAFC)
However, since this device is operated under highly acidic environment, only Pt-based metal catalyst can be used as a corrosion-tolerant electrode catalyst. As a result, prevailing of this technology is difficult due to high cost. Recently, solid-alkaline fuel cell (SAFC) using anion exchange membrane (AEM) has drawn much attention (Fig 1 right) 1. Since most of metal is stable under alkaline condition, various types of metal catalysts including non-noble metal Ni, Co catalysts can be used as electrode catalysts. Furthermore, high-energy density liquid fuel such as formate or hydrazine in addition to hydrogen gas can be used in this fuel cell by designing suitable catalysts.
However, practical high-performance AEMs tolerant in highly alkaline condition have not been developed in SAFC while highly durable perfluorosulfonic acid PEM like Nafion have been already developed in PEFC.
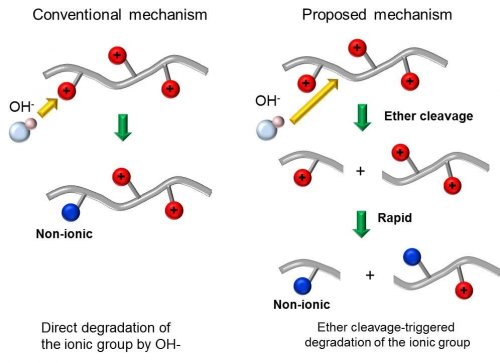
In our laboratory, alkaline degradation mechanism of general aromatic AEM was analyzed by using model compound for the purpose of developing highly durable AEM in alkaline solution. It is well-known that anion conductivity of AEM gradually decreases after soaking in concentrated alkaline solution. Therefore, degradation of the membrane is thought to be derived from the decomposition of anion exchange group (Fig 2 left). As a result, development of highly durable anion exchange group is thought to be critical for enhancing alkaline durability of AEMs. However, from careful analysis of the degradation mechanism, it was confirmed that the ether linkage in the main-chain of AEMs is decomposed firstly and this triggered the decomposition of the anion exchange group of AEMs because the resulting structure after ether cleavage is extremely unstable (Scheme 1 and Fig2 right)2,3. Since degradation of general aromatic AEM proceeds through the decomposition of the main-chain, simple design for enhancing durability of anion exchange group is not effective but rather design of highly stable backbone is important for obtaining durable AEMs.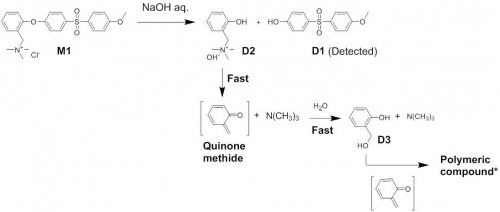
Scheme 1 Alkaline degradation mechanism of polyethersulfone AEM by using low-molecular model compound
Fig 2 Alkaline degradation mechanism of general aromatic AEM
Polyphenylene-based membrane, composed of only aromatic component without ether or any hetero atom is a promising AEM having very strong backbone. However, preparation of well-defined membrane of this type of materials is generally difficult due to low solubility and low mechanical property, derived from highly rigid backbone and strong intermolecular interaction.
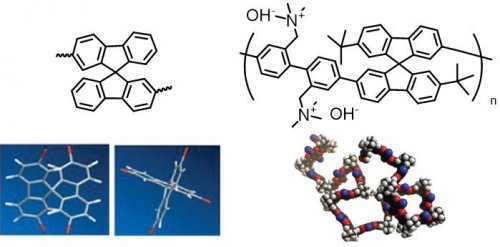
In this research, we have developed spirobifluorene-based AEMs having spiro-segment in the backbone4. Three dimensionally twisted polymer can be obtained by introducing the spirobifluorene in the backbone in proper manner, since two fluorene ring twists orthogonal at the center in this segment (Fig 3). As a result, highly soluble polyelectrolyte membrane can be prepared. The synthesized AEM is highly tolerant against swelling and shows 86mS/cm high OH- conductivity (80 oC in water).
Fig 3. Chemical structure and three dimensional structure of spirobifluorene and spirobifluorene-based AEM
Alkaline stability and oxidative stability of the developed polymer was evaluated in 1M NaOH aq. at 80 oC and Fenton's solution (3wt% H2O2, 3ppm FeSO4) at 60 oC, respectively.
From 1H-NMR spectra of the polymer before and after the stability test, it was confirmed that both the backbone and the anion exchange group are stable in both accelerating durability tests. Highly durable nature of the developed AEM is derived from the strong backbone without any degradation-prone cite like hetero atom, since generally applied quaternary ammonium group is introduced as an anion exchange group in this polymer5.
Finally, the synthesized AEM was applied for both an ionomer and a protective layer of generally-applied polyelectrolyte membrane and formate liquid fuel cell was prepared. As a result, 212 mW/cm2 performance was obtained in this fuel cell, which is among the highest value of the world in this system (Fig 4 left). Furthermore, we for the first time demonstrated that the fabricated formate fuel cell is successfully operated for more than one week without degradation of the performance (Fig 4 right).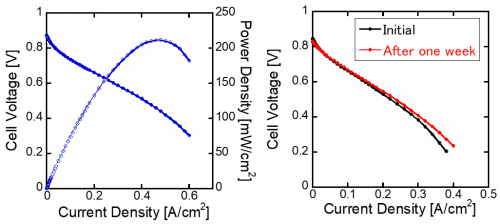
Fig 4. Potassium formate fuel cell fabricated with the developed AEM: Fuel cell performance (left) and its durability for one week (right)
Further enhancement of the performance and durability of the fuel cell is possible in the future by improving the developed AEM in this research.
Reference
- 1) Shoji Miyanishi, Takeo Yamaguchi, ''Nanocarbons for Energy Conversion: Supramolecular Approaches'' Springer, 2019, Chapter 14. p.309-350
- 2) S. Miyanishi, T. Yamaguchi, Phys. Chem. Chem. Phys., 18, 12009-12023 (2016).
- 3) S. Miyanishi, T. Yamaguchi, New J. Chem., 41(16), 8036-8044 (2017)
- 4) S. Miyanishi, T. Yamaguchi, J. Mater. Chem. A, 7, 2219-2224 (2019)
- 5) H. P. R. Graha, S. Ando, S. Miyanishi and T. Yamaguchi, Chem. Commun., 10820-10823 (2018)