Latest Research
- 2019.08.01
Characterization of basicity of lattice oxygen on proton-form zeolites using IR with CO2 probe method
Catalytic reactions over solid acid catalysts are caused by proton-donation from the surface acidic OH groups as active sites, and this mechanism is similar to the case of homogeneous acid-catalyzed reactions. On the other hand, the contribution of lattice oxygen atoms to acid-catalyzed reactions in a concerted manner has been suggested. In the case of ethanol dehydration over proton-form zeolites, ethoxy groups on zeolites were first formed by dehydration of adsorbed ethanol molecules and acidic OH groups. Then, ethene is formed by the decomposition of ethoxy groups regenerating acidic OH groups (Fig. 1), where hydrogen atoms in methyl groups of ethoxy species are selectively abstracted1).
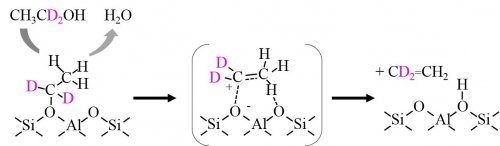 Fig. 1 Reaction scheme of ethanol dehydration over ZSM-5 zeolite.
Fig. 1 Reaction scheme of ethanol dehydration over ZSM-5 zeolite.
This process indicates that the acidic OH groups and basic lattice oxygens work as acid-base pair sites (Fig. 2), i.e. the former is attributed to the acid catalysis, and the latter to the base catalysis. Therefore, the characterization of basicity of lattice oxygen on solid acid catalysts is very important.
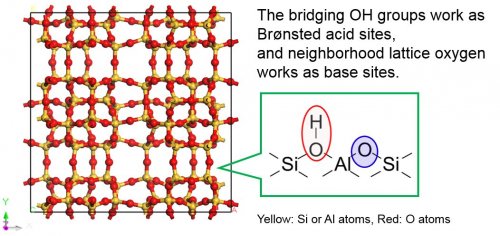 Fig. 2 Framework structure of MFI type zeolite and schematic illustration of acid sites.
Fig. 2 Framework structure of MFI type zeolite and schematic illustration of acid sites.
In the conventional method using infrared (IR) spectroscopy, pyrrole and chloroform have been used as probe molecules to characterize the basicity of zeolites2). Some disadvantages are claimed for these methods; the multi-point adsorption structure and large molecule size of probes. In our laboratory, we attempted various probe molecules in order to verify the presence of base sites on proton-form zeolites based on the above problems. As a result, the basicity in lattice oxygens pairing with acidic OH groups was probed by CO2 adsorption3). The experiment could be performed by using in-situ IR cell connected to a conventional closed gas-circulation system at room temperature. Fig. 3 shows difference IR spectra of adsorbed CO2 species on ZSM-5 zeolite, where the presence at least three types of basic lattice oxygen with difference property were confirmed. In addition, the experiment of CO2 adsorption on zeolites with different topology revealed that the types of basic lattice oxygen were affected by the zeolite topology.
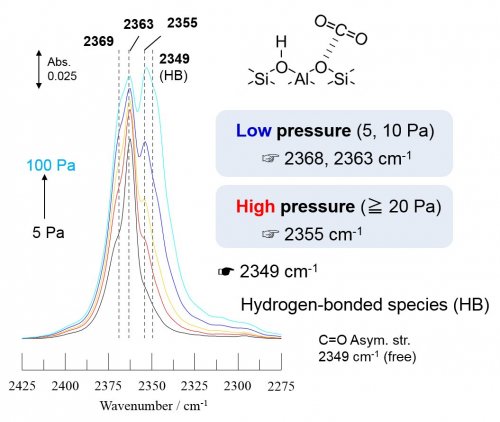 Fig. 3 Difference IR spectra of adsorbed CO2 species on ZSM-5 at room temperature.
Fig. 3 Difference IR spectra of adsorbed CO2 species on ZSM-5 at room temperature.
Moreover, the amount of adsorbed CO2 species linearly decreased with increasing the consumption of acidic OH groups by NH3 adsorption. Thus, the presence of the acid-base pair sites (Fig. 4) was evidenced. In conclusion, we succeeded in experimentally demonstrating the basic lattice oxygen on solid acid catalysts for the first time.
 Fig. 4 Model for CO2 adsorption on proton-form zeolite.
Fig. 4 Model for CO2 adsorption on proton-form zeolite.
- 1) J. N. Kondo, H. Yamazaki, R. Osuga, T. Yokoi, T. Tatsumi, J. Phys. Chem. Lett. 6 (2015) 2243.
- 2) J. C. Lavalley, Catal. Today 27 (1996) 377.
- 3) R. Osuga, T. Yokoi, J. N. Kondo, J. Catal. 371 (2019) 291.



