Latest Research
- 2019.11.05
- Nishiyama-Miura Group
Photodynamic therapy utilizing drug delivery systems based on precisely designed functional polymers
In recent years, photodynamic therapy (PDT) has attracted attention as a minimally invasive method for treating tumors. PDT consists of accumulation of photosensitizers (drugs that produce highly reactive oxygen species in response to light) in a tumor, and subsequent photoirradiation, thereby killing tumor cells in a light-selective manner (Fig. 1) (1). In addition to the direct damage to cancer cells by reactive oxygen species, PDT can induce damage to tumor-associated blood vessels to block the blood flow and prevent the supply of nutrients to tumor cells. Furthermore, because PDT can activate immune cells, the combination therapy of PDT and immunotherapy is expected to be a promising approach to efficiently treat tumors. Currently, photosensitizers such as Photofrin and Laserphyrin have been used clinically, and excellent treatment results have been obtained for several tumors including early lung cancers and malignant brain tumors. Meanwhile, there are still tumors within which these photosensitizers cannot selectively accumulate. Expansion of the application of PDT requires new technologies to efficiently deliver photosensitizers to such tumors. In this regard, we have been working on the development of drug delivery systems based on precisely designed functional polymers. Here, we introduce the following two functional polymer-conjugates as the drug delivery systems for PDT.
- 1. Polymer conjugates showing isothermal phase transition behavior in response to intratumoral low pH
- 2.Polymer conjugates targeting both tumor-associated blood vessels and tumor cells
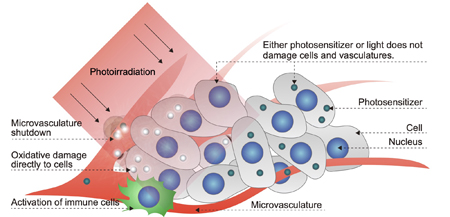 Figure1. Mechanism of photodynamic therapy (PDT) (1).
Figure1. Mechanism of photodynamic therapy (PDT) (1).
- 1. Polymer-conjugates showing isothermal phase transition behavior in response to intratumoral low pH
In general, intratumoral pH is lower than that of normal tissues, ranging from 6.8 to 7.3. We thought that photosensitizers can be selectively delivered to the tumor by utilizing a polymer that is hydrophilic in blood and normal tissues (at pH 7.4) to avoid interaction with cells and biological components and is hydrophobic in low pH environments to actively interact with cells. Although low pH-responsive drug delivery systems have been developed by many researchers, only few drug delivery systems could respond to very small pH changes (e.g., from pH 7.4 to pH 6.8~7.3). We thus developed a functional polymer that changes its property from hydrophilic to hydrophobic in response to a slightly acidic pH at a constant temperature (37 ° C), using poly(N-isopropylacrylamide) (PNIPAAm) which is a temperature-responsive polymer that exhibits a lower critical solution temperature (LCST *) (Fig. 2) (2). This polymer, termed P(NIPAAm/AIPAAm-PMM), was hydrophilic and difficult to be taken up by cells at physiological pH (7.4), but it was efficiently internalized into cells when pH decreased (Fig. 3). Experiments using a mouse subcutaneous tumor model showed that P(NIPAAm/AIPAAm-PMM) could selectively accumulate in the tumor. We have recently investigated the therapeutic potential of photosensitizer-conjugated P(NIPAAm/AIPAAm-PMM), and it has exhibited strong PDT effect.
*LCST: Polymers exhibiting LCST are hydrophobic at temperatures above LCST and hydrophilic at temperatures below LCST.
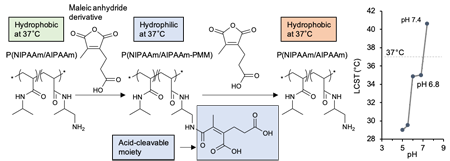 Figure 2. The functional polymer changing its property from hydrophilic to hydrophobic in response to a slightly acidic pH (P[NIPAAm/AIPAAm-PMM]) (2).
Figure 2. The functional polymer changing its property from hydrophilic to hydrophobic in response to a slightly acidic pH (P[NIPAAm/AIPAAm-PMM]) (2).
 Figure 3. The cover art illustrating that P(NIPAAm/AIPAAm-PMM) interacts with tumor cells in response to intratumoral pH (2).
Figure 3. The cover art illustrating that P(NIPAAm/AIPAAm-PMM) interacts with tumor cells in response to intratumoral pH (2).
- 2. Polymer conjugates targeting both tumor-associated blood vessels and tumor cells
To further enhance tumor selectivity, it is important to design molecules that actively interact with the tumor. In addition, we thought that it is useful to target not only tumor cells but also tumor-associated blood vessels at the same time in order to effectively utilize the therapeutic mechanism of PDT. Here, we developed a polymer conjugate targeting αvβ3 integrin, which has been reported to be overexpressed on tumor-associated blood vessels and many tumor cells (Fig. 4) (3). Specifically, this polymer conjugate has multiple cyclic RGD peptides (cRGDs) showing strong affinity for αvβ3 integrin in the side chain with the backbone of biocompatible poly (ethylene glycol)-poly(L-glutamic acid); the hydrophilic photosensitizer, IRDye 700DX, is conjugated to the terminal of poly(ethylene glycol). The polymer conjugate could be selectively and efficiently internalized into cells overexpressing αvβ3 integrin. Owing to this function, in in vivo study, the polymer conjugates exhibited little accumulation within normal tissues, but efficiently accumulated in the subcutaneous tumor in a mouse. Furthermore, as the number of cRGDs conjugated to the polymer increased, the polymer conjugates could retain in the tumor for a longer time and more preferentially accumulated in the tumor-associated blood vessels, leading to the dramatic enhancement of the PDT effect. This study suggests that targeting both tumor cells and tumor-associated blood vessels may be a useful strategy in PDT.
As described above, we have developed technologies to deliver photosensitizers selectively to a target tumor with diverse approaches, based on precisely designed polymers. Since actual human tumors vary widely and have various phenotypes, it is important to establish a variety of treatments including our drug delivery systems and select the appropriate method for each patient.
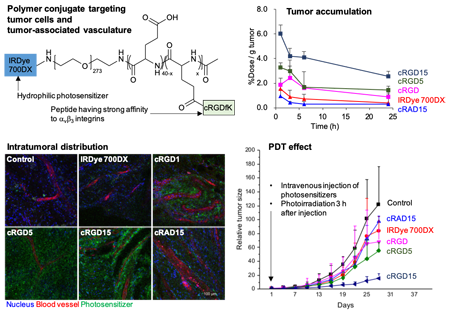 Figure 4. Polymer conjugates targeting both tumor-associated blood vessels and tumor cells (3).
Figure 4. Polymer conjugates targeting both tumor-associated blood vessels and tumor cells (3).
References
1. T. Nomoto, N. Nishiyama, Design of drug delivery systems for physical energy-induced chemical surgery. Biomaterials 178, 583-596 (2018).
2. S. E. Muttaqien, T. Nomoto, H. Takemoto, M. Matsui, K. Tomoda, N. Nishiyama, Poly( N-isopropylacrylamide)-Based Polymer-Inducing Isothermal Hydrophilic-to-Hydrophobic Phase Transition via Detachment of Hydrophilic Acid-Labile Moiety. Biomacromolecules 20, 1493-1504 (2019).
3. X. Dou, T. Nomoto, H. Takemoto, M. Matsui, K. Tomoda, N. Nishiyama, Effect of multiple cyclic RGD peptides on tumor accumulation and intratumoral distribution of IRDye 700DX-conjugated polymers. Sci. Rep. 8, 8126 (2018).



