Latest Research
- 2019.12.01
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
Bottom-up approach to ion selectivity of K+ channels by cold ion trap technique
The K+ channels are the membrane protein that allows K+ to pass through a lipid membrane. Na+, which is more abundant in nature than K+, is not allowed to permeate a lipid membrane via the K+ channels despite its smaller ionic radius than K+. A question arises as to why Na+ with a smaller ion radius cannot penetrate the K+ channels. Much research has been conducted to answer this question. MacKinnon's group successfully crystallized a K+ channel and found that an amino acid sequence GYGVT (G: glycine, Y: tyrosine, V: valine, T: threonine) called "selectivity filter" is coordinated to K+ (Figure 1) [1]. Because this coordination structure is close to the hydration structure of K+, the energy loss by dehydration of K+ could be sufficiently compensated by interaction with the selectivity filter. However, the process of the exclusion of Na+ from the selectivity filter in competition with hydration has only been proposed by theoretical calculation [2] and has never been experimentally observed. In this study, the complexes between a partial peptide GYG in the selectivity filter and an alkali metal ion (Na+, K+) were introduced into the gas phase to investigate the geometric structures using cold ion trap spectroscopy [3].
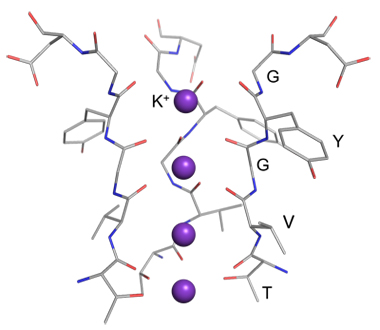 Figure 1 Crystal structure of the selectivity filter in the K+ channels.
Figure 1 Crystal structure of the selectivity filter in the K+ channels.
GYG-M+ (M = Na+, K+) was produced in the gas phase by electrospray ionization, mass-selected by a quadrupole mass spectrometer, and trapped by a cold ion trap at 4 K [4]. Hydrogen molecules introduced into the ion trap were attached to GYG-M+. Hydrogen-dissociated fragment ions are generated when an ion absorbs an infrared (IR) light. IR absorption spectra were obtained by monitoring the fragment yield as a function of wavenumber of an IR laser.
Figure 2 shows the IR absorption spectra of GYG-Na+ and GYG-K+ in the gas phase. In the spectra of both complexes, strong bands were observed in 3400-3500 cm−1 attributed to the NH stretching modes of GYG. By using an advanced spectroscopic technique, these bands were assigned for each conformer (Figure 2). It was revealed that the bidentate structure where two COs are coordinated to M+ (O/O, O/O') and the tridentate structure where M+ is additionally coordinated by the benzene ring (O/O/R) were observed as a coexisting conformer. Although the structural motifs of the conformers observed are identical in both complexes, the abundance among the conformers is different. The O/O structure is dominant in GYG-K+, whereas the abundance of the O/O/R structure is significantly increased in GYG-Na+. The O/O structure is similar to the structure found in the selectivity filter, but the O/O/R structure cannot be realized in the selectivity filter. The large contribution of the O/O/R structure in GYG-Na+ suggests that the (O/O-type) coordination of Na+ to the selective filter is unstable.
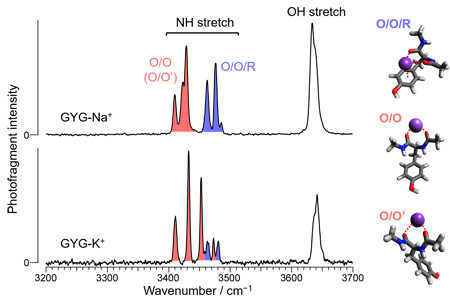 Figure 2 IR absorption spectra of GYG-M+ (M = Na+, K+) with structural assignment.
Figure 2 IR absorption spectra of GYG-M+ (M = Na+, K+) with structural assignment.
[References]
- 1. Zhou, Y.; Morals-Cabral, J. H.; Kaufman, A.; MacKinnon, R. Nature 2001, 414, 43.
- 2. Varma, S.; Sabo, D.; Rempe, S. B. J. Mol. Biol. 2008, 376, 13.
- 3. Ishiuchi, S.; Sasaki, Y.; Lisy, J. M.; Fujii, M. Phys. Chem. Chem. Phys. 2019, 21, 561.
- 4. Ishiuchi, S.; Wako, H.; Kato, D.; Fujii, M. J. Mol. Spectrosc. 2017, 332, 45.



