Latest Research
- 2020.02.01
- Nakamura-Okada Group
Raman cell imaging with boron cluster molecules
Boron neutron capture therapy (BNCT) is a cancer cell-selective radiotherapy. In this therapy, a tumor-localizing drug containing non-radioactive boron isotope (10B) is introduced to a tumor, and a nuclear reaction is induced by irradiating the tumor with an epithermal neutron beam having an appropriate energy. For achieving high therapeutic efficacy, it is important to accumulate boron drugs in tumor cells while suppressing the accumulation of boron drugs in normal tissue. To evaluate the efficacy of boron drugs, it is crucial to measure the distribution and dynamics of boron drugs in tissues and cells, but it is still challenging.
Fluorescence microscopy, one of the most powerful tools for the evaluation of cellular behavior, is capable of real-time molecular imaging with nanoscale resolution. However, fluorescent labeling may alter the cellular uptake rate of boron drugs, because their hydrophobic and electrostatic properties lead to undesired interactions to the biomolecules. And fluorescence intensity is often affected by intracellular and extracellular environments.
Raman microscopy can visualize the distribution of organic and biological molecules in the cell without any fluorescent probes by detecting the Raman scattering of the specific vibrational modes of the molecules. However, in the case of the small target molecules, their local distribution is difficult to visualize because the Raman bands of cellular components often overlap with and mask those of small molecules of interest. So, Raman probes are required to possess unique Raman bands at 1800-2800 cm-1, which is a specific region where all the natural cellular components do not generate the Raman bands (called cellular-Raman silent region).
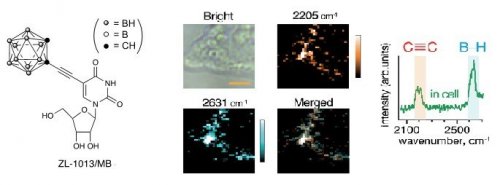
We focused on B-H stretching vibrations of Raman bands at 2570 cm-1, which is within the cellular-Raman silent region,1 and found that carborane derivatives and closo-dodecaborate derivatives showed Raman bands in cellular-Raman silent region. ZL-1013/MB, an ortho-carborane derivative, has an internal alkyne moiety, thus can be performed for Raman imaging of both B-H and C≡C2-4 stretching vibrations of Raman bands in the cells.5
Figure 1. A Raman spectrum and Raman images of HeLa cells incubated with ZL-1013/MB
In this method, the imaging speed of Raman microscopy is limited to 1 s/pixel because of the weak intensity of spontaneous Raman scattering, making it difficult to conduct imaging experiments in various conditions. So, we demonstrate the direct imaging of cellular uptake of boron cluster compound detected by stimulated Raman scattering (SRS) microscopy.6,7
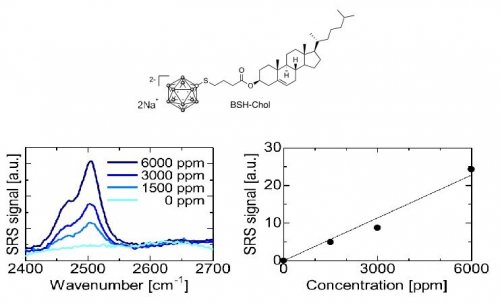
As a boron drug, we synthesized closo-dodecaborate derivative, disodium BSH-cholesterol (BSH-Chol) shown in Fig 2. Then we examined its Raman responses in the wavenumber region from 2400 to 2700 cm−1. We can see that the BSH has a Raman peak at 2505 cm−1, which is assigned to the B-H stretching mode. We can also confirm a linear dependence of the SRS signal on the concentration as shown in Fig. 2
Figure 2. Characteristics of BSH-Chol
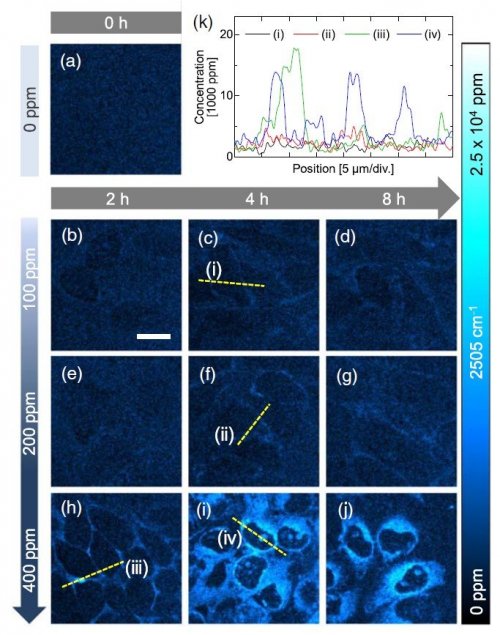
Then we conducted SRS imaging of HeLa cells cultured with BSH-Chol at different concentration. The results of SRS imaging are summarized in Fig. 3. The concentration of BSH-Chol molecules was increased to 15,000 ppm in the cytoplasm and nucleoli, suggesting that molecules are concentrated by a factor of >35. These results show that SRS imaging can be used to quantitatively analyze the spatial distribution of BSH-Chol after cellular uptake.8
Figure 3. SRS images of BSH-Chol in HeLa cells.
The present method allows the direct imaging of cellular uptake of BSH without fluorescent labeling. Importantly, BSH is detected with the B-H stretching vibrational mode in the cellular silent region, where endogenous molecules in cells do not have Raman signals.
To summarize, we have demonstrated the imaging analysis of cellular uptake of boron-containing molecules for BNCT. We anticipate that Raman imaging of boron-based drugs will be useful for evaluating the efficacy of drug delivery to develop highly specific and efficient drug delivery to cancer cells for BNCT.
[References]
- 1) M. Tarr´es, E. Canetta, C. Vi˜nas, F. Teixidor and A. J. Harwood, Chem. Commun., 2014, 50, 3370-3372.
- 2) H. Yamakoshi, K. Dodo, M. Okada, J. Ando, A. Palonpon, K. Fujita, S. Kawata and M. Sodeoka, J. Am. Chem. Soc., 2011, 133, 6102-6105.
- 3) H. Yamakoshi, K. Dodo, A. Palonpon, J. Ando, K. Fujita, S. Kawata and M. Sodeoka, J. Am. Chem. Soc., 2012, 134, 20681-20689.
- 4) L. Wei, F. H. Hu, Y. H. Shen, Z. X. Chen, Y. Yu, C. C. Lin, M. C. Wang and W. Min, Nat. Methods, 2014, 11, 410-412.
- 5) M. Mochizuki, S. Sato, S. Asatyas, Z. J. Le´snikowski, T. Hayashi and H. Nakamura, RSC Adv, 2019, 9, 23973-23978.
- 6) J. X. Cheng and X. S. Xie, Science, 2015, 350, aaa8870.
- 7) Y. Ozeki, W. Umemura, Y. Otsuka, S. Satoh, H. Hashimoto, K. Sumimura, N. Nishizawa, K. Fukui, and K. Itoh, Nat. Photon., 2012, 6, 845-851.
- 8) T. Asai, H. Liu, Y. Ozeki, S. Sato, T. Hayashi and H. Nakamura, Appl. Phys. Express, 2019, 12, 12004
【Acknowledgements】
This work was achieved in the collaboration with the research groups of Associate Professor Tomohiro Hayashi, School of Materials and Chemical Technology, Tokyo Tech, Associate Professor Yasuyuki Ozeki, Department of Electrical Engineering and Information Systems, The University of Tokyo, and Professor Zbigniew J. Lesnikowski, Polish Academy of Sciences and Tokyo Tech World Research Hub Initiative (WRHI).