Latest Research
- 2020.05.11
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
Light-independent redox regulation allows cyanobacteria to perform both photosynthesis and nitrogen-fixation during the day
[Background]
Various enzyme activities are highly regulated to respond to environmental changes in cells. In photosynthetic organisms, thiol-disulfide redox system allows the light-dependent activation or conclusioninactivation of enzymes. In this system, thioredoxin (Trx) is the key protein by transferring the reducing power from photosynthetic electron transport chain to its target proteins.
Cyanobacteria, photosynthetic microorganisms, are thought to be the ancestors of the chloroplasts in plants. A filamentous cyanobacterium Anabaena sp. PCC 7120 (Anabaena 7120) can directly fix atmospheric nitrogen into ammonium during the day. This reaction is performed in the specialized cell named heterocyst which is differentiated from the vegetative cell under nitrogen-depleted conditions. In heterocysts, NADPH generated via the oxidative pentose phosphate pathway (OPPP) is used as a source of reducing power for the nitrogen-fixation. Amino acids are synthesized using the resulting ammonia and then exported to the adjacent vegetative cells. In the previous study, we have shown that the redox states of Trx target proteins are changed in response to not only light conditions but also nitrogen conditions in Anabaena 71201).
[Objective]
Glucose 6-phosphate dehydrogenase (G6PDH) catalyzes the first step of OPPP and plays a pivotal role in NADPH production in heterocysts. We have shown that G6PDH is deactivated by Trx-dependent reduction of its activator protein named OpcA under light conditions1). However, OpcA was mostly present as the oxidized form in the absence of combined nitrogen source even under light conditions, implying that OpcA is oxidized, and G6PDH activity is maintained in heterocysts1). In order to understand the light-independent redox regulation of G6PDH via OpcA, we investigated the differences in the redox regulation system between vegetative cells and heterocysts in Anabaena 7120.
[Results]
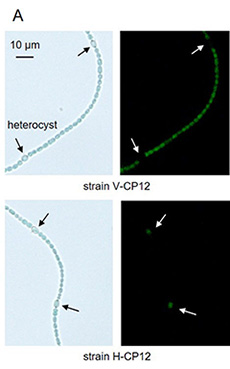
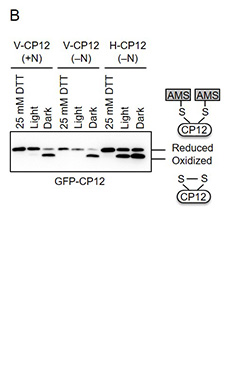
The recent study suggested that OpcA is reduced in vegetative cells but oxidized in heterocysts in the light1). To investigate the redox status of Trx target proteins in vegetative cells and heterocysts, GFP-tagged CP12 was expressed in vegetative cells and heterocysts, respectively. CP12 is one of Trx target proteins that was already used as a core protein for the redox sensor2). The strains expressing GFP-tagged CP12 in vegetative cells or heterocysts were designated as V-CP12 and H-CP12, respectively (Fig. 1A). The redox states of GFP-tagged CP12 in these strains were measured using thiol-modifying reagent AMS, and it was found that the redox state of the protein was changed in response to light in V-CP12 strain irrespective of nitrogen conditions, but not in H-CP12 strain3) (Fig. 1B). While GFP-tagged CP12 was fully reduced in vegetative cells in the light, more than half of GFP-tagged CP12 was maintained as the oxidized form even under light conditions3) (Fig. 1B). These results suggest that Trx target proteins are regulated in a light-independent manner in heterocysts.
Fig. 1. Redox state of Trx target proteins in vegetative cells and heterocysts under nitrate-added conditions (+N) or nitrogen-depleted conditions (-N).
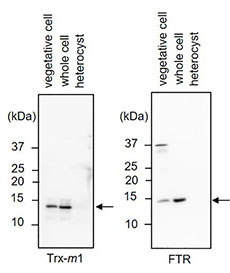
We assumed that differences in the composition of redox regulation system may result in differences in the redox states of Trx target proteins between vegetative cells and heterocysts. We therefor examined the protein amounts of ferredoxin-Trx reductase (FTR) and Trx-m1, an efficient electron donor for OpcA, in these cells. FTR and Trx-m1 were detected in vegetative cell fraction and whole cell fraction, but not in heterocyst fraction (Fig. 2), indicating that the drastic decrease of the amounts of these proteins in heterocysts3).
Fig. 2. Western blot analysis of FTR and Trx in vegetative cells, whole cells, and heterocysts.
[Conclusion]
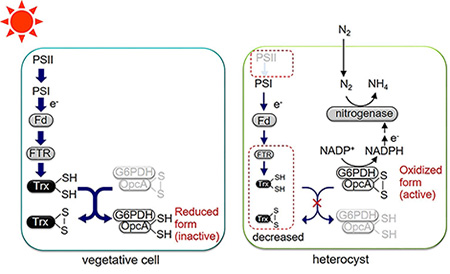
In this study, we show that Trx target proteins are regulated in a light-independent manner in heterocysts. Due to the lack of photosystem II and the limited expression of FTR and Trx-m1 in heterocysts, Trx target proteins are supposed to be partially oxidized even under light conditions (Fig. 3). This light-independent regulation may play a fundamental role in nitrogen-fixation in the light.
Fig. 3. Proposed model of redox cascade in vegetative cells and heterocysts.
Further information is available at https://doi.org/10.1093/jxb/erz561.
[References]
1 .Mihara, S., Wakao, H., Yoshida, K., Higo, A., Sugiura, K., Tsuchiya, A., ... Hisabori, T. (2018). Thioredoxin regulates G6PDH activity by changing redox states of OpcA in the nitrogen-fixing cyanobacterium Anabaena sp. PCC 7120. Biochemical Journal. https://doi.org/10.1042/BCJ20170869
2. Sugiura, K., Yokochi, Y., Fu, N., Fukaya, Y., Yoshida, K., Mihara, S., & Hisabori, T. (2019). The thioredoxin (Trx) redox-state sensor protein can visualize Trx activities in the light-dark response in chloroplasts. Journal of Biological Chemistry, jbc.RA119.007616. https://doi.org/10.1074/jbc.ra119.007616
3. Mihara, S., Sugiura, K., Yoshida, K., & Hisabori, T. (2020). Thioredoxin targets are regulated in heterocysts of cyanobacterium Anabaena sp. PCC 7120 in a light-independent manner. Journal of Experimental Botany, 71(6), 2018-2027. https://doi.org/10.1093/jxb/erz561