Latest Research
- 2020.07.01
- Yamamoto-Imaoka Group
Development of "Superatoms": unique sub-nano sized metal particles
The superatom is the metal particle composed of several atoms, and has attracted much attention since it shows intrinsic properties of element completely different from the original constituent elements in the particle.1 This feature can be explained by the element-like symmetry in the electronic orbitals of the superatom (Fig. 1), and thus, expecting the possibility for the substitution of certain element by superatoms.
In spite of such high expectations, application of the superatoms for materials is not simple because the synthetic process is exceptionally difficult, which requires high precision in assembling atoms and structuring a particle at atomicity-level. In literature, the synthesis of such ultrafine metal particles has been reported using the laser vaporization technique under a high vacuumed condition.2 However, due to its low production rate, synthesis in large quantity with this technique is technically unrealistic. Therefore, the development of synthetic method of superatoms in solution phase has been expected to open the way for mass preparation essential for their applications.
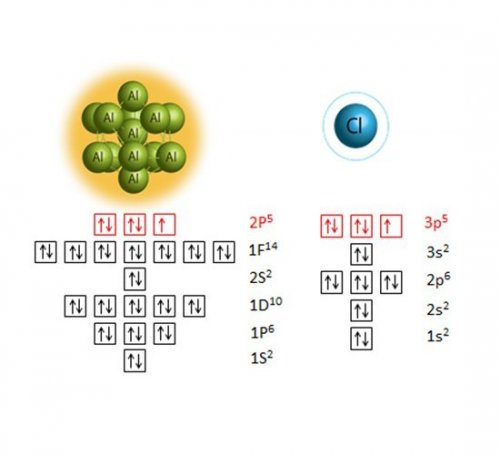 Fig. 1. Electronic orbitals of the superatom and atom
Fig. 1. Electronic orbitals of the superatom and atom
Here we report the synthesis of the sub-nano sized particles with typical metals. We focused on chemical species of group 13 (boron, aluminum and gallium), group 14 (tin) and group 15 (bismuth).3 Their sub-nano sized metal particles were successfully prepared, and their unique properties, such as superatomicity, were demonstrated (Fig. 2).4,5
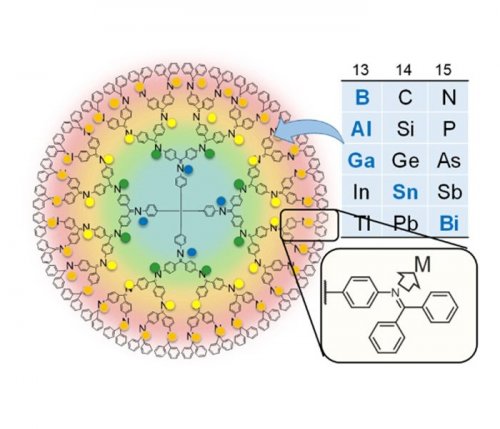 Fig. 2. Stepwise complexation of typical metal units in the DPA
Fig. 2. Stepwise complexation of typical metal units in the DPA
A π-conjugated dendrimer with phenylazomethine skeleton (DPA: Dendritic poly-phenylazomethines) was utilized for the synthesis of these superatoms.6 The DPA molecule has two major features: one is that metals can be complexed at the imine sites, and the other is that the basicity of the imine is stronger for the inner sites and decreases toward the outer layers. These features facilitate a stepwise complexation of metal salts from the central imines toward outer imines of the DPA, and the number of metals accumulated in the DPAs can be controlled by the amount of added metal salts. The following chemical reduction of multi-complexed DPA provides atomicity controlled metal particles within the DPA.7 In particular, assembly of 13 metals was achieved by using the dendrimer molecule with an addition of one pyridine part at the core position.8 This pyridine-modified DPA enabled the synthesis of 13-atom magic numbered metal particles (Fig.3).
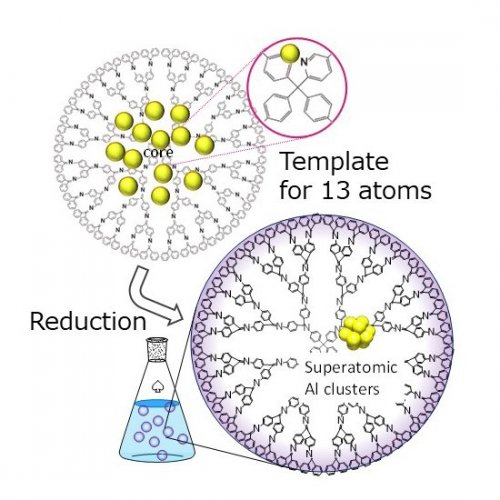 Fig. 3. Solution-phase synthesis of the superatom with the dendrimer
Fig. 3. Solution-phase synthesis of the superatom with the dendrimer
In this research, we have succeeded in the solution-phase synthesis of 13-atom metal particles composed of 13th group elements (Al13, Ga13, etc.), which are representative examples of superatoms that express the property of halogens.4 In addition, their basic properties were clarified by photoelectron spectroscopy, electron microscopic observation and electrochemical measurements. Furthermore, we have succeeded in expanding variations focusing on new functions by using various typical metals such as tin and bismuth.
The series of results demonstrated in our research are based on the peculiarity of the sub-nano sized materials. Considering further development of this research field, investigation of new analytical methods is inevitable. Now, we are focusing on the direct observation of the sub-nano sized particles by an electron microscope,9 electrochemical measurement, and spectroscopic analysis with enhanced sensitivity.10 We expect that such pioneering researches will open the design and creation of new functional sub-nano sized materials.
References
| 1. | a) D. E. Bergeron, P. J. Roach, A. W. Castleman Jr, N. O. Jones and S. N. Khanna, Science 2005, 307, 231-235; b) S. N. Khanna and P. Jena, Phys. Rev. B 1995, 51, 13705; etc. |
| 2. | W. D. Knight, K. Clemenger, W. A. Deheer, W. A. Saunders, M. Y. Chou and M. L. Cohen, Phys. Rev. Lett. 1984, 52, 2141-2143. |
| 3. | a) T. Tsukamoto, T. Kambe, A. Nakao, T. Imaoka and K. Yamamoto, Nature Commun. 2018, 9, 3873; b) T. Kambe, A. Watanabe, T. Imaoka and K. Yamamoto, Angew. Chem. Int. Ed. 2016, 55, 13151-13154; c) T. Kambe, T. Imaoka and K. Yamamoto, Chem. Eur. J. 2016, 22, 16406-16409. |
| 4. | a) T. Kambe, N. Haruta, T. Imaoka and K. Yamamoto, Nature Commun. 2017, 8, 2046; b) T. Kambe, A. Watanabe, M. Li, T. Tsukamoto, T. Imaoka and K. Yamamoto, Adv. Mater. 2020, 32, 1907167. |
| 5. | a) T. Kambe, S. Imaoka, R. Hasegawa, T. Tsukamoto, T. Imaoka, K. Natsui, Y. Einaga and K. Yamamoto, J. Inorg. Organomet. Polym. 2019, 30, 169-173. b) T. Kambe, R. Hosono, T. Imaoka and K. Yamamoto, J. Photopolym. Sci. Technol. 2018, 31, 311-314. |
| 6. | a) K. Yamamoto, M. Higuchi, S. Shiki, M. Tsuruta and H. Chiba, Nature 2002, 415, 509-511; b) O. Enoki, H. Katoh and K. Yamamoto Org. Lett. 2006, 8, 569-571. |
| 7. | a) K. Yamamoto, T. Imaoka, M. Tanabe and T. Kambe, Chem. Rev. 2020, 120, 1397-1437; b) K. Yamamoto and T. Imaoka, Acc. Chem. Res. 2014, 47, 1127. |
| 8. | H. Kitazawa, K. Albrecht and K. Yamamoto, Chem. Lett. 2012, 41, 828-830. |
| 9. | T. Imaoka, T. Toyonaga, M. Morita, N. Haruta and K. Yamamoto, Chem. Commun. 2019, 55, 4753-4756. |
| 10. |
A. Kuzume, M. Ozawa, Y. Tang, Y. Yamada, N. Haruta and K. Yamamoto, Sci. Adv. 2019, 5, eaax6455. |



