Latest Research
- 2020.07.31
Organometallic Molecular Wires For Molecular Devices: Effects of Metal Fragments
Integrated circuits have become an indispensable tool for modern society. On the other hand, the cost of developing high-performance electronic circuits based on conventional silicon semiconductor technology increases year by year. Thus, alternative methods for electronic circuits are widely explored. Molecular electronics is expected to construct high-performance electronic circuits because the size of the circuits can be miniaturized into the nanometer scale (Fig. 1). Furthermore, various functions can be created using synthetic organic chemistry methods. On the other hand, the expected functions cannot be fully exploited due to the considerable resistance between the organic material and the electrode. One of the reasons is large energy gap between the electrode's Fermi level and the frontier orbitals of the organic molecular wires. [1]
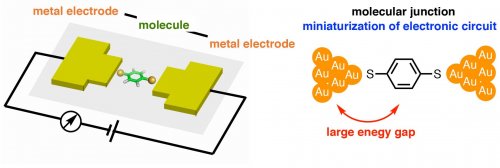
Fig. 1. Molecular electronics and a example of molecular junction
On the other hand, we have been studying mixed-valence chemistry using organometallic complexes with metal-carbon bonds. The characteristics of strong metal-carbon bonds realize efficient intermetallic electron transfer through the bridging ligands. [2] We hypothesized that electron-rich organometallic complexes could be 'doped' (incorporated) into organic molecular wires to give a high affinity between metal electrodes and molecular wires.
We then developed an organometallic molecular wire with a bifunctional Ru tetraphosphine fragment and pyridine anchor groups (Fig. 2a). [3] The single-molecule conductance was measured by the STM break-junction method (Fig. 2b). In this method, an Au tip is moved in and out to form and break the junction. By immersing the substrate in a solution containing molecules, single-molecule junctions accidentally form. The single-molecule conductance of the molecular wires is statistically determined. The organometallic molecular wires exhibit a conductance 2.5-fold higher than that of the organic molecular wires. Considering that the single-molecule conductance exponentially decreases with molecular length elongation, the introduction of organometallic complexes was shown to be effective in improving the conductance.
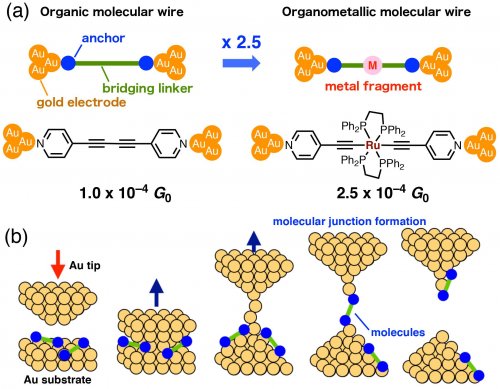 Fig. 2. (a) Organic and organoetalllic molecular wires with pyridine anchor groups. (b) The STM break-junction method.
Fig. 2. (a) Organic and organoetalllic molecular wires with pyridine anchor groups. (b) The STM break-junction method.
To develop molecular wires with higher single-molecule conductance, we then turned our attention to polyynes (Fig. 3a). Polyynes are theoretically predicted to have high conductance.[4] They are, however, known to be thermodynamically unstable and often explosive due to their high self-reactivity. Therefore, it was not easy to use them as a conduction material. On the other hand, the metallapolyyne, a polyyne "doped" with metal complexes, can be expected to increase their stability as the bulky ligands prevent self-reaction (Fig. 3b). [5]
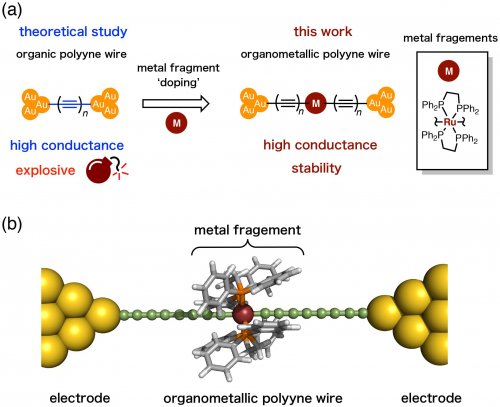 Fig. 3. (a) Organic and organometallic polyyne wires. (b) A model of an organometallic polyyne junction.
Fig. 3. (a) Organic and organometallic polyyne wires. (b) A model of an organometallic polyyne junction.
For the formation of covalent bonds between the molecule and the electrodes, molecular wires functionalized with gold complexes were designed and subjected to STM-BJ measurements. The results showed that the performance was about 100 and 6 times higher than those of the organic polyyne wires with pyridine and thioether anchor groups, respectively (Fig. 4a). It was also found that the conductance was 80 times higher than that of the organometallic molecular wire with pyridine anchor groups. The distance-conductance plot revealed that one of the factors was the low contact resistance between the molecule and the electrode.
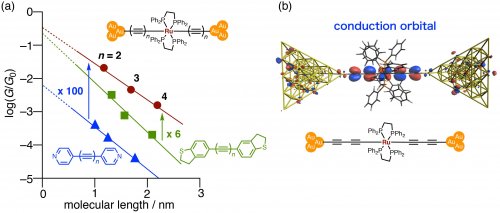 Fig. 4. (a) A length-conductance plot. (b) A conduction orbital of an organometallic molecular wire.
Fig. 4. (a) A length-conductance plot. (b) A conduction orbital of an organometallic molecular wire.
The hybrid density functional theory and non-equilibrium Green's function (DFT-NEGF) analysis was carried out to investigate the high conductance mechanism. The molecular conduction orbitals are located near the electrode's Fermi energy levels (Fig. 4b). There is large energy gap between the molecular orbitals and the electrodes in the polyynes without metal complexes. Therefore, the "doping" of the metal complexes is the key to the high conductance.
A new molecular wire of a different metal fragment was designed to elucidate the "doping" effect of the metal complexes. A cationic, electron-deficient rhodium molecular wire was synthesized (Fig. 5).[6] Single-molecule conductance measurements revealed a conductance of 6.7 x 10-3 G0, which was reduced one-third compared to those of ruthenium polyyne molecular wires with the same chain length. The result indicates that the electron-rich metal fragments have significant interactions between the metal electrode and the metal complex, suggesting that conductance can be controlled by tuning the electronic interactions between them.
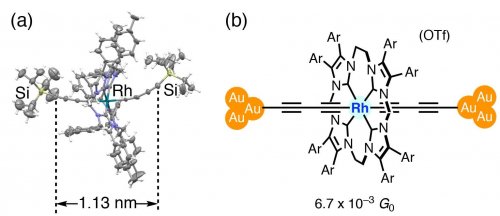 Fig. 5. (a) X-ray structure of a precursor of the cationic rhodium molecular wire. (b) The rhodium metallapolyyne junction.
Fig. 5. (a) X-ray structure of a precursor of the cationic rhodium molecular wire. (b) The rhodium metallapolyyne junction.
Future Prospects
The present study demonstrates that the introduction of metal complexes into organic molecular wires leads to high conductance due to significant electronic interactions between the metal electrode and metal complexes. On the other hand, the decay constant, which is a deteriorating factor of conductance with increasing molecular length, is comparable to those of the organic polyyne wires. Thus, our future goals are to develop molecular wires with high conductance even at several nanometers lengths and to explore functions in response to external stimuli.



