Latest Research
- 2020.09.02
- Fukushima-Shoji Group
New design concept of a molecular memory with an alternating circular array of dipolar rotors and rotation suppressors
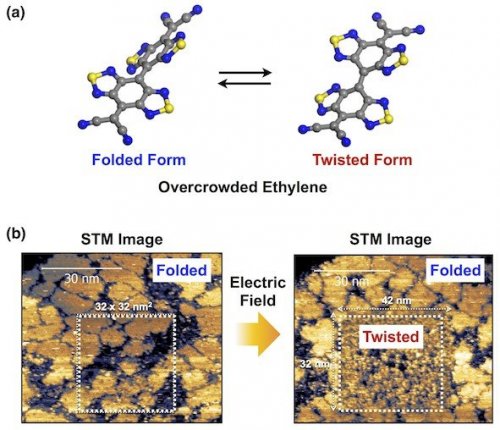
One of major goals in molecular electronics is to construct nano-electric circuits using single molecules or self-assembled monolayers (SAM).[1] For this purpose, molecular elements are required to possess at least two stable states with different electronic properties, which are interconvertible to one another by the application of external stimuli. Recently, our group has succeeded in constructing a SAM capable of switching electrical conductivity by the application of electric fields[2] using an overcrowded ethylene molecule possessing two conformational states (folded and twisted, Fig. 1a).[3] On Au(111), folded isomers were found to form a thermally stable SAM due to the formation of a two-dimensional network by specific intermolecular interactions (Fig. 1b). Interestingly, upon application of an electric field, the folded form in the SAM was converted into a twisted form, resulting in a great enhancement in the electrical conductivity of SAM (Fig. 1b). While the overcrowded ethylene-based SAM is promising for molecular switch and memory, a problem with this system is that further chemical modification of the molecule is impossible.
Fig. 1. Conductance switching of overcrowded ethylene-based SAM. (a) Molecular structure and (b) STM images of the SAM before and after application of an electric field.
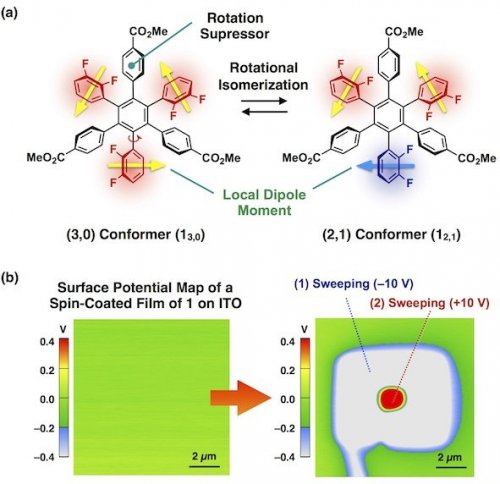
  In the present study, we have proposed a new structural element for molecular switches based on a hexaarylbenzene building block (Fig. 2a).[4] Molecule 1 possesses two rotational isomers (13,0 and 12,1) due to the orientation of the difluorophenyl groups, which are substituted at the 1,3,5-positions of the central benzene ring (Fig. 2a). Ester group-appended aryl groups, substituted at the 2,4,6-positions, are expected to suppress the free rotation of the difluorophenyl groups and thus endow substantial stability with the rotational isomers. In fact, each rotational isomer exhibited sufficient thermal stability in solution at room temperature. However, at higher temperatures, the interconversion between 13,0 and 12,1 took place, which was confirmed by 19F NMR spectroscopy. Interestingly, amorphous thin films obtained by spin-coating of a solution of 1 onto an ITO substrate can change the surface potential in response to the sign of applied electric fields, and the resulting surface states were retained for a long time (Fig. 2b). In sharp contrast, the corresponding crystalline films of 1, obtained after thermal annealing of the amorphous film, did not show such surface potential changes under identical conditions. This is most likely due to close molecular packing in the crystalline state, which eliminates a free volume for ring rotation.
Fig. 2. (a) Design of a new molecular switch (1) based on a hexaarylbenzene building block. (b) Surface potential map of a spin-coated amorphous film of 1 on ITO before and after application of electric fields.
  Various functional groups can be introduced to 1 through the ester groups of the aryl group at the 2,4,6-positions. We are currently investigating electric-field response properties of SAMs and single-molecular junctions using a derivative of 1 carrying surface-anchoring groups. The present molecular design concept that enables control of the rotation barriers of dipolar rotating units is expected to be applicable to the development of various stimuli-responsive materials.
| [1] | L. Sun, Y. A. Diaz-Fernandez, T. A. Gschneidtner, F. Westerlund, S. Lara-Avila, K. Moth-Poulsen, Chem. Soc. Rev., 2014, 43, 7378. |
| [2] | S. Fujii, M. Koike, T. Nishino, Y. Shoji, T. Suzuki, T. Fukushima, M. Kiguchi, J. Am. Chem. Soc. 2019, 141, 18544. |
| [3] | T. Suzuki, T. Fukushima, T. Miyashi, T. T. Tsuji, Angew. Chem. Int. Ed. Engl. 1997, 36, 2495. |
| [4] | T. Miyazaki, Y. Shoji, F. Ishiwari, T. Kajitani, T. Fukushima, Chem. Sci. 2020, 11, 8388. |