Latest Research
- 2020.10.01
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
- Ueda-Kitaguchi Group
Quick conversion of antibodies into fluorescent sensors - Easy and sensitive detection of various small molecules
Antibody-based immunoassays are used in various fields such as clinical diagnosis, environmental analysis, and food hygiene as a specific and sensitive detection method for trace substances in samples. In particular, immunochromatography, a type of immunoassay, has recently been attracting attention as a rapid diagnostic technique for antigens and antibodies in infectious diseases. However, it is difficult to achieve both sensitivity and simplicity of measurement, and the realization of a rapid, easy to use immuno-sensor has been awaited.
A few years ago, we had succeeded in constructing a Quenchbody (Q-body), an immunosensor protein that enables highly sensitive quantification of antigens simply by fluorescently labeling the N-terminal region of the antibody, mixing it with a sample and measuring the fluorescence a few minutes later (Fig. 1) [Ref. 1]. In the Q-body, while in the absence of the antigen, the dye is quenched by the photoinduced electron transfer PeT from tryptophan residues in the antibody, upon adding the antigen the dye is moved out from the antibody and emits fluorescence [Ref 2]. We have succeeded in detecting many antigens, from small molecules to proteins, with high sensitivity by changing the linker length and introducing multiple dyes. However, in many cases, these require time-consuming gene cloning of antibodies, recombinant protein expression in E. coli and chemical modification of dyes, and the performance of resultant Q-body could not be predicted before construction.
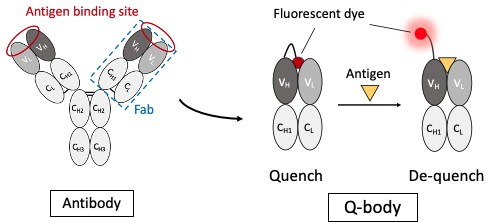
Fig. 1 Quenchbody, which is an antibody-fragment biosensor labeled with fluorescent dye. The attached dye such as TAMRA is quenched by Trp residues, and recovers fluorescence upon antigen binding. The method provides rapid easy target detection.
In this study, we have developed a probe (PM Q-probe) that can easily convert many antibodies, including commercially available ones, into fluorescent biosensors using antibody-binding protein Protein M (PM) [Ref. 3]. It binds strongly to a wide range of antibodies at the light chain and disrupts the immune system by structurally blocking their binding to antigens [Ref. 4]. We hypothesized that the C-terminus of the PM is close to the antigen-binding site upon antibody binding and that the binding of the antigen is not interfered with by small molecules, and thus we constructed a probe containing a dye introduced into the C-terminus of the PM via a short linker and named it PM Q-probe (Fig. 2). This Q-probe was found to have similar properties to Q-body, as its fluorescence was quenched by 50% when mixed with osteocalcin-recognizing antibody Fab fragments or full-length IgG antibodies, which are bone disease markers used as models, and its fluorescence was restored when antigen osteocalcin or its C-terminal fragment was added. Even more interestingly, the complex with IgG showed higher antigen detection sensitivity than the recombinantly constructed Fab (Fig. 3).
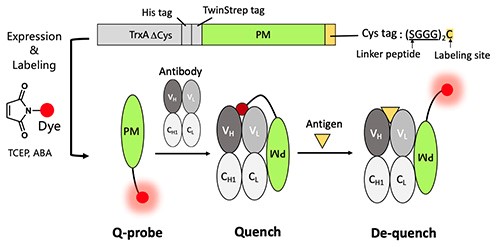
Fig. 2. Quenchprobe (Q-probe), which is an antibody binding protein labeled with fluorescent dye at the C-terminus. The scheme for construction, and expected quenching and de-quenching mechanisms are shown.
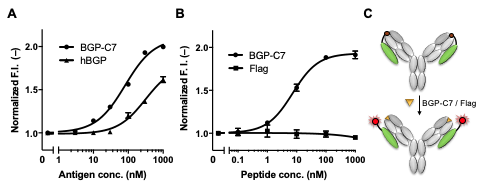
Fig. 3. Detection of BGP-C7 antigen by recombinant Fab (A) and native IgG (B,C). hBGP is the full length human BGP. Flag peptide is a negative control. While lower response for hBGP is observed in A, this is also observed for recombinant Q-body.
Next, we measured the concentrations of several small biomarkers such as progesterone, male hormone and thyroid hormone T4, all used in blood diagnostics, using IgGs including commercially available ones. We could measure these substances with sufficiently high sensitivity that covers the normal range in the blood of healthy individuals. We also succeeded in determining the concentration of T4 extracted from serum with ethanol. In other words, we were able to determine the total T4 concentration, including T4 bound to proteins, in the blood (Fig. 4). However, direct measurement of serum samples was difficult because of the dissociation of Q-probe/IgG complexes caused by antibodies in the serum.
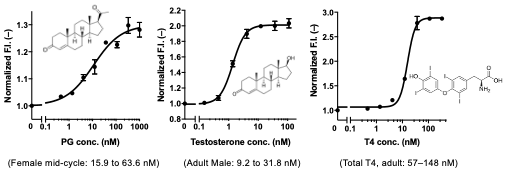
Fig. 4. Detection of steroid hormones (progesterone, testosterone, and thyroxine) by Q-probe/IgG complex. Normal concentration ranges in serum are also shown.
Therefore, using four antibodies to cortisol, a stress hormone, as a starting material, we selected a promising clone by Q-probe and constructed a cortisol Q-body by genetic engineering of the genes from antibody-producing cells. The constructed Q-body showed a fluorescence response similar to that of the Q-probe/IgG complex, and furthermore, the performance of the Q-body in 50% serum was comparable or even better than that of the Q-probe in buffer (Fig. 5). These results indicate that Q-probe can be used to select antibodies suitable for Q-bodies, and the resulting Q-bodies can be used for direct measurement of antigens in serum.
These results suggest that this technology may expand the range of application of Q-bodies and make the detection of various molecules much simpler than in the past.
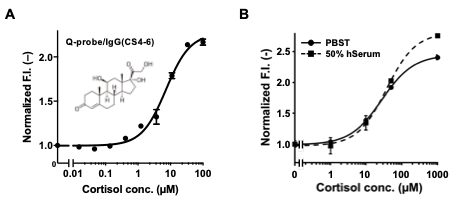
Fig. 5. Conversion of an anti-CS IgG to a Q-body. Detection of cortisol by Q-probe/IgG complex (A) and scFv-type Q-body (B). In B, detection in 50% human serum is also shown.
References
| [1] | R. Abe, H. Ohashi, I. Iijima, M. Ihara, H. Takagi, T. Hohsaka, H. Ueda. J. Am. Chem. Soc. 2011, 133, 17386-17394. |
| [2] | H. Ohashi, T. Matsumoto, H.-J. Jeong, J. Dong, R. Abe, and H. Ueda, Bioconj. Chem., 2016, 27, 2248-2253. |
| [3] | J. Dong, C. Miyake, T. Yasuda, H. Oyama, I. Morita, T. Tsukahara, M. Takahashi, H.-J. Jeong, T. Kitaguchi, N. Kobayashi and H. Ueda Biosens. Bioelectron. 2020, 165, 112425, Pat. P. WO/2018/147018 (Tokyo Tech). |
| [4] | Grover, R. K., et al.: Science, 2014, 343, 656-661. |



