Latest Research
- 2021.01.28
- Yamaguchi-Kuroki Group
Development of high performance and durable membrane-electrode-assembly for pure water solid alkaline water electrolyzer
By 2050, the world's population will reach more than 9 billion, and the global economy and energy consumption are expected to grow 4 times and 1.8 times larger, compared to those in 2012, respectively. Meanwhile, in order to prevent global warming, CO2 emissions must be reduced to less than half of the current levels. Therefore, to break away from the dependence of fossil fuels, energy policies in countries have recently shifted to increasing the utilization of CO2-emission-free, renewable energy, such as solar and wind power. In this situation, hydrogen energy system draws growing interest. In this system, renewable energy with unstable supply will be converted to hydrogen carrier (chemical energy), which enables large-scale energy storage and transportation. The produced hydrogen can be converted to electricity whenever and wherever necessary by using fuel cell or hydrogen gas turbine.
 |
|||
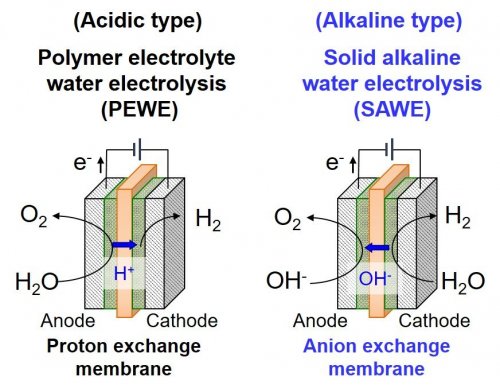
| Fig. 1. | Schematic illustration of water electrolysis systems using solid polymer electrolyte membranes. |
||
A common hydrogen production technology is water electrolysis (Fig. 1), in which hydrogen and oxygen can be produced from water that is abundant in the world. Among the water electrolysis methods, polymer electrolyte water electrolysis (PEWE) using proton exchange membranes has been partially commercialized. Although PEWE system has several advantages such as high conversion efficiency, high cost remains an issue since noble metals (e.g., Pt) are necessary for electrode catalysts and bipolar plates to prevent the corrosion and dissolution of metals under highly acidic and high-voltage operations. The cost of bipolar plates using noble metals accounts for most of the total cost of the PEWE system. To overcome this issue, solid alkaline water electrolysis (SAWE) using anion exchange membranes, which operates in an alkaline conditions, has recently attracted much attention. Since most metals are stable under an alkaline environment, various non-noble metals such as Ni or Co can be used for bipolar plates and electrode catalysts, leading to significant cost reductions in water electrolysis systems. However, conventional polyelectrolytes for anion exchange membranes and ionomers suffer from low alkaline durability, and therefore no realistic membranes and ionomers have been developed so far for the application to SAWE.
Our group has clarified the degradation mechanism of conventional aromatic polyelectrolytes to design and develop chemically durable membranes and ionomers.[1] Our study indicates that polyphenylene-based polymers consisting of all-aromatic linkages without degradation prone-sites such as ether linkages, heteroatoms, etc., are promising to improve chemical durability of polyelectrolytes under alkaline conditions.[2] However, the intermolecular entanglement of such polymers less forms due to their rigid backbone and low molecular weight, resulting in low mechanical strength.
 |
|||
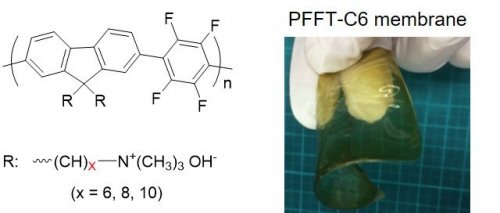
| Fig. 2. | Chemical structure of the anion conducting polyelectrolytes of PFFT-Cx (x = 6, 8, 10), and appearance of the PFFT-C6 membrane |
||
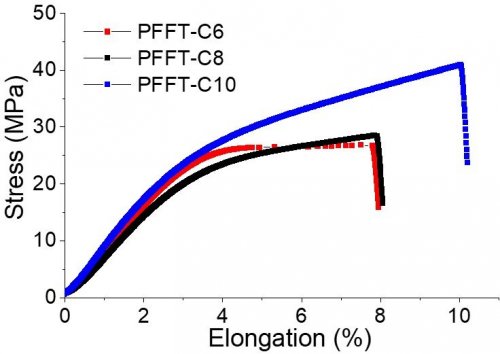
In this work, new series of all-aromatic anion conducting polyelectrolytes (Poly(fluorene-alt-tetrafluorophenylene)-trimethyl Ammonium: PFFT-Cx shown in Fig. 2) with high molecular weight (weight-average molecular weight = 20 kDa) were successfully synthesized by using the C-H activation polymerization method. The preparation of these polymers are easy and mass-producible because of only three synthetic steps: monomer synthesis, polymerization, and introduction of ion exchange groups. In spite of their high molecular weight, the developed polymers are soluble in various solvents owing to the introduction of long alkyl side chains.The membranes prepared from these polymers with high molecular weight showed high tensile strength (25-42 MPa), which are comparable to the standard Nafion membrane of PEWE (Fig. 3). In addition, the membranes exhibited high OH− conductivity over 100 mS/cm (at 70 °C) due to high ion exchange capacity (around 3 meq/g). The high alkaline stability of the membranes was also confirmed by the fact that no sign of degradation was observed in the accelerating alkaline stability test at 80 °C in 8 M NaOH aqueous solution for one week.[3]
 |
|||
| Fig. 3. | Stress-strain curve of the PFFT-Cx membranes. |
||
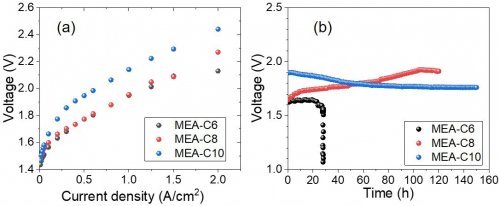
Next, the membrane-electrode-assemblies (MEA-C6, MEA-C8, and MEA-C10) using the developed polyelectrolytes with different length of alkyl side chains (PFFT-C6, PFFT-C8, and PFFT-C10 shown in Fig. 2) were prepared, and the performance and durability of the MEAs were tested for pure water SAWE. Figure 4a shows the water electrolysis performances (current-voltage curves) operating at 80 °C with pure water feed. To date, most SAWE have been operated in highly concentrated alkaline solution feed to ensure sufficiently high OH− conduction in MEAs. However, the MEAs using our developed polyelectrolytes of PFFT-Cx successfully achieved high SAWE performances even at pure water operations due to high OH− conduction of PFFT-Cx without any alkaline feed. In addition, it was found that, as the length of the side chains of PFFT-Cx became longer, the OH− conductivity of PFFT-Cx lowered, resulting in the slight reduction of the initial water electrolysis performances of the MEAs. On the other hand, longer side chains of PFFT-Cx improved mechanical strength of the membranes due to less swelling, resulting in higher durability of the MEAs (Fig. 4b).Especially, MEA-C10 prepared using PFFT-C10 with sufficiently high OH− conductivity and mechanical strength successfully demonstrated stable pure water SAWE operation at high temperature of 80 °C for la long period of 150 h. This study is the first report of pure water SAWE with both high water electrolysis performance and stability.[4]
| Fig. 4. | (a) Water electrolysis performances of the MEAs prepared using our PFFT-Cx polymers for pure water SAWE at 80 °C. (b) Durability tests of the MEAs operating at 80 °C and 200 mA/cm2. |
||
The results obtained in this study will greatly contribute to realizing the practical application of SAWE. In addition, our developed polyelectrolyte materials with excellent chemical durability and mechanical strength is also expected to be applied to other energy devices.
References
| [1] | S. Miyanishi and T. Yamaguchi, Phys. Chem. Chem. Phys., 18, 12009-12023 (2016) |
| [2] | H. P. R. Graha, S. Ando, S. Miyanishi and T. Yamaguchi, Chem. Commun., 54, 10820-10823 (2018) |
| [3] | S. Miyanishi and T. Yamaguchi, Poly. Chem., 11, 3812-3820 (2020) |
| [4] | R. Soni, S. Miyanishi, H. Kuroki and T. Yamaguchi, ACS Appl. Energy Mater., in press, DOI: 10.1021/acsaem.0c01938 |