Latest Research
- 2021.02.08
Facile preparation of NaNbO3 perovskite under non-hydrothermal conditions
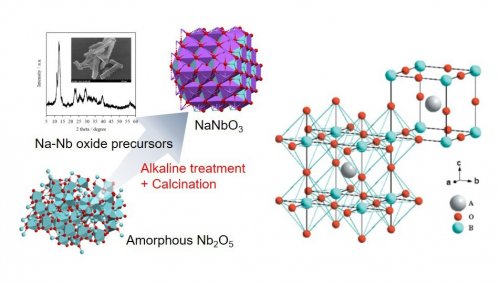
Perovskite-type oxides represented by a general formula ABO3 consist of the structure described in Fig. 1, they and are a family of inorganic materials practically used as ferroelectric and piezoelectric ceramics. While the control of their crystal phase and morphology is required for the further efficiency, they are synthesized under severe conditions and/or with high energy input by methods represented by solid state reactions, hydrothermal synthesis, molten salt method, mechanochemical treatment and so on. The synthesis under milder conditions would enable the facile control of the structure and the morphology. NaNbO3 perovskite, whose phase diagram has not been completely clarified due to the presence of various phases and their phase transitions, was found to be prepared under soft conditions; stirring in an alkaline solution followed by calcination in air (Fig. 1)1), which is introduced below.
 |
|||
| Fig. 1 | Non-hydrothermal synthesis of NaNbO3 and the crystal structure of an ideal cubic perovskite |
||
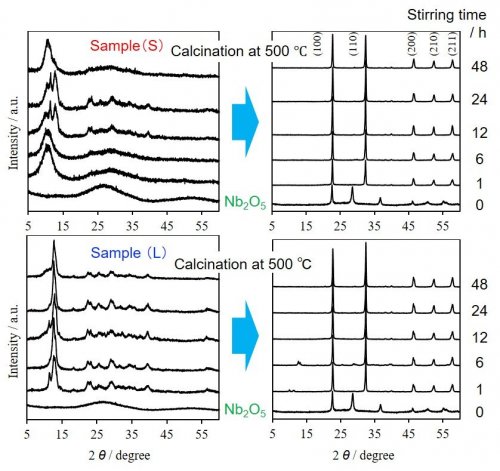
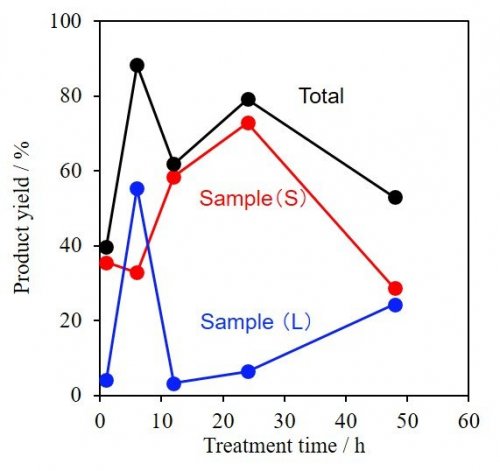
Amorphous Nb2O5 (1.5 g) was added to an NaOH aqueous solution (2.0 mol·L-1, 50 mL) and stirred for 1-24 h. This method is much milder than the conventional alkaline treatment (NaOH 10.0 mol·L-1 and above) and alkaline molten salt methods. The white powder, Sample (S), was obtained by filtration, washing and drying. In addition, Sample (L) was collected by diluting the filtrate by water. X-ray diffraction (XRD) patterns of all samples are shown in Fig. 2 (left), and the product yield in Fig. 3. In Fig. 3 the initial product yield of Sample (S) was less than 40 %, and the drastic increase in the yield of Sample (L) was observed at 6 h, indicating the dissolution of amorphous Nb2O5 at the beginning of the reaction. An amorphous hallo pattern at around 10 ° was observed in XRD data of Sample (S) at this stage, while Sample (L) indicated some evident peaks attributed to crystalline structures. The extension of the reaction time produced peaks at 10-15 ° assigned to any crystalline phases in Sample (S), but it finally resulted again to amorphous at 48 h by dissolution, which was found in Fig, 3. The attribution of observed crystalline structures in Sample (S) and Sample (L) to Na2Nb2O6·H2O2), Na7(H3O)Nb6O19·14H2O and its anhydrate3, 4) is now under confirmation.
 |
|||
| Fig. 2 | XRD patterns of reaction products of amorphous Nb2O5 in aqueous NaOH solution |
||
 |
|||
| Fig. 3 | Change of product yield during alkaline treatment |
||
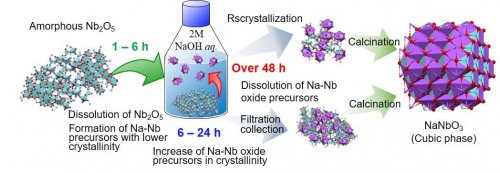
All XRD patterns of Sample (S) and Sample (L) after calcination at 500 °C (Fig. 2, right) consisted of distinct peaks are ascribed to cubic NaNbO3 crystal phase. That is, various amorphous and crystalline precursors, which were generated from alkaline treatment of amorphous Nb2O5, resulted in cubic NaNbO3 perovskite by simple calcination in air (Fig. 4). In addition, Sample (L) consisted of rod-like particle as seen in its SEM image (Fig. 1), which implies the possibility of the morphology regulation of perovskite materials due to the soft syntheticmethod1).
 |
|||
| Fig. 4 | Proposed mechanism of NaNbO3 perovskite formation |
||
The application of the present method to the synthesis of other ABO3 perovskite oxides with various combinations of alkaline or alkaline earth metals and transition metals are explored, and mechanisms of the formation of various precursors and their phase transitions are to be investigated.
References
| 1) | R. Osuga, Y. Hiyoshi, T. Yokoi, J. N. Kondo, J. Sold State Chem., 295 (2021) 121891. |
| 2) | W. Mu, X. Li, G. Liu, Q. Yu, X. Xie, H. Wei, Y. Jian, Dalton Trans. 45 (2016) 753-759. |
| 3) | S. Yamazoe, T. Kawawaki, T. Imai, T. Wada, J. Ceram. Soc. Jpn. 118 (2010) 741-744. |
| 4) | S. Yamazoe, K. Shibata, K. Kato, T. Wada, Chem. Lett. 42 (2013) 380-382. |



