Latest Research
- 2021.03.01
- Yoshizawa-Sawada Group
Development of Useful Nano Tools for Water Solubilization
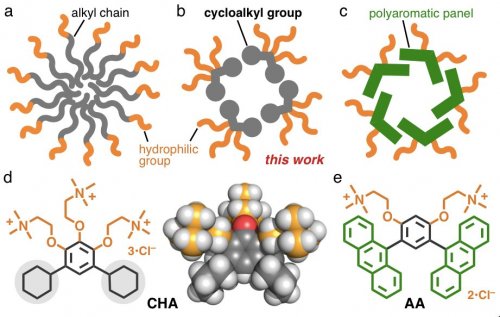
Linear alkanes, which are photo- and electrochemically inactive, act as useful building blocks for various natural and artificial compounds. Cell membranes and micelles are representative molecular assemblies, composed of multiple amphiphiles having linear alkyl chains (Figure 1a). In contrast, the usability of "cyclic" alkanes for biological and synthetic amphiphiles has been obscure so far, except for cholic acid. The rigidity of the cyclic frameworks is higher than that of the acyclic ones and the photoreactivity of cycloalkanes is lower than that of aromatic frameworks. Therefore, the rational incorporation of cycloalkyl groups into amphiphiles is expected to develop a novel micelle system with unique functions. Here we report the preparation of a cycloalkane-based micelle (Figure 1b) and its unusual host properties toward metal-complexes in water.[1]
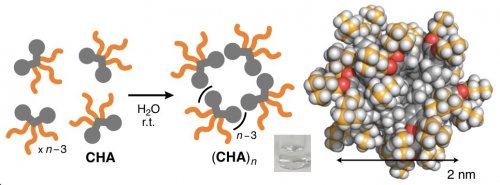
On the basis of anthracene-based bent amphiphile AA (Figure 1e), reported previously by our group, we replaced the aromatic panels by cycloalkyl groups and synthesized new amphiphile CHA bearing three hydrophilic groups (Figure 1d). Cycloalkane-based micelle (CHA)n was instantly formed in water from amphiphiles CHA through the efficient intermolecular interactions (Figure 2). The spectroscopic analyses of the product suggested the selective formation of a spherical (CHA)12 structure with a diameter of ~2 nm. The present micelle provides weak UV-visible absorption bands, in contrast to aromatic micelle (AA)n (Figure 1c).
 |
|||
| Figure 2 | Schematic representation of the quantitative formation of micelle (CHA)n in water and its three-dimensional structure (n = 12). | ||
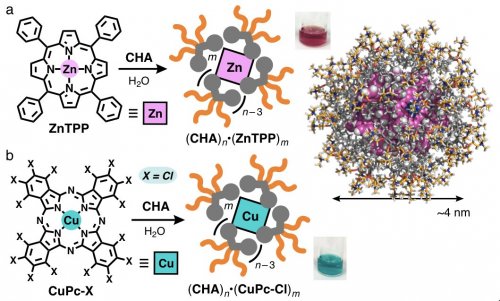
Next we revealed that cycloalkane-based micelle (CHA)n exhibits excellent uptake ability toward highly hydrophobic metalloporphyrin dye ZnTPP in water, as compared with previous micelles. For the uptake, a mixed solid of CHA and ZnTPP was ground using an agate mortar and pestle. The resultant solid was dissolved in water and the subsequent filtration of the suspension gave rise to host-guest complex (CHA)n •(ZnTPP)m as a clear purple solution (Figure 3a). The dye encapsulation by (CHA)n was more than 6 and 2 times higher than that by usual micelle and aromatic micelle, respectively. The product size analyses indicated the formation of a spherical host-guest complex, accommodating multiple ZnTPP dyes (m = ~9), with a diameter of ~4 nm (Figure 3a, right).
 |
|||
| Figure 3 | The encapsulation and water-solubilization of a) ZnTPP and b) CuPc-Cl dyes by micelle (CHA)n in water. The three-dimensional structure of host-guest complex (CHA)45•(ZnTPP)9. | ||
Micelle (CHA)n displayed the selective uptake of substituted metallophthalocyanines in water. Simple mixing of CHA and perchlorinated metallophthalocyanine CuPc-Cl (X = Cl) via manual grinding led to the efficient formation of green host-guest complex (CHA)n •(CuPc-Cl)m in water (Figure 3b). In sharp contrast, neither non-substituted metallophthalocyanine CuPc-H (X = H) nor perfluorinated one CuPc-F (X = F) was incorporated into (CHA)n under various conditions.
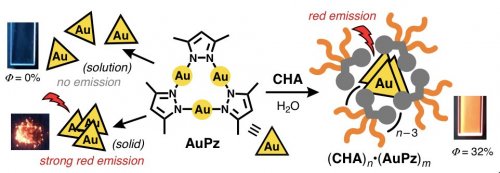
Finally, a water-soluble host-guest complex with strong red emission was successfully obtained from CHA and a trinuclear gold complex. AuPz is known for being highly emissive only in the solid state, due to the intermolecular gold-gold interactions. The solution-state emission of gold complexes is quite rare. Micelle (CHA)n efficiently encapsulated multiple AuPz compounds in water (Figure 4, right). Notably, the resultant host-guest complex (CHA)n •(AuPz)m emitted strong red phosphorescence (>30% emission quantum yield) upon UV-light irradiation, whereas no emission was observed from free AuPz in organic solvent (Figure 4, left).
 |
|||
| Figure 4 | Schematic representation of the emission properties of AuPz in solution and in the solid state, and upon encapsulation by micelle (CHA)n in water. | ||
In summary, to explore unusual host functions of micellar systems, we have designed and synthesized a cycloalkane-based amphiphilic compound. The water-solubilization of various planar metal-complexes (metal = zinc, copper, and gold) was succeeded upon facile encapsulation by a new cycloalkane-based micelle in this work.[1] On the basis of the present and our previous studies, we would like to further develop novel micellar systems as useful nano tools in water.
| References | |
| [1] | M. Hanafusa, Y. Tsuchida, K. Matsumoto, K. Kondo, M. Yoshizawa*, Nature Commun., 2020, 11, 6061 (https://www.nature.com/articles/s41467-020-19886-4). |
| Recent our publications | |
| [2] | K. Niki, T. Tsutsui, M. Yamashina, M. Akita, M. Yoshizawa*, Angew. Chem. Int. Ed. 2020, 59, 10489-10492. |
| [3] | H. Dobashi, L. Catti, Y. Tanaka, M. Akita, M. Yoshizawa*, Angew. Chem. Int. Ed. 2020, 59, 11881-11885. |
| [4] | N. Kishida, K. Matsumoto, Y. Tanaka, M. Akita, H. Sakurai, M. Yoshizawa*, J. Am. Chem. Soc. 2020, 142, 9599-9603. |
| [5] | K. Ito, T. Nishioka, M. Akita, A. Kuzume, K. Yamamoto, M. Yoshizawa*, Chem. Sci. 2020, 11, 6752-6757. |
| [5] | T. Tsutsui, L. Catti, K. Yoza, M. Yoshizawa*, Chem. Sci. 2020, 11, 8145-8150. |
| [7] | K. Kudo, T. Ide*, N. Kishida, M. Yoshizawa*, Angew. Chem. Int. Ed. 2021, 60, in press. |