Latest Research
- 2021.08.02
- Nishiyama-Miura Group
Polyphenols-based self-assembled nanomachines for potential protein delivery.
In recent years, bioactive proteins have attracted significant attention as therapeutic agents due to their specific pharmacological effects for intractable diseases such as cancer disease. However, due to the enzymatic degradation and phagocytosis by immune cells as foreign substances, the proteins showed a poor blood circulation, and their efficacy has been limited.1
Various protein delivery systems have been reported that encapsulate proteins in nanoparticles to reduce immunogenicity, avoid degradation, and prolong blood retention.1 For example, liposomes composed of lipid bilayers, and polymeric nanoparticles can physically encapsulate proteins or adsorb them on their membrane, which improve their pharmacokinetics and pharmacological effects. In addition to these nanoparticle-based delivery systems, we have recently developed novel nanoparticles with a facile preparation in aqueous solution and applied these nanoparticles to protein delivery systems.2
 |
|||
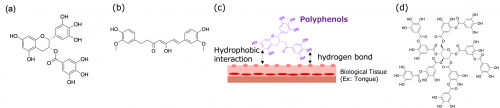
| Figure. 1 | Chemical structure of (a) epigallocatechin gallate, (b) curcumin, and (d) tannic acid (TA). (c) Illustration of astringent effect of polyphenols. | ||
To construct the novel nanoparticles for protein delivery, we focused on polyphenols, which is one of the familiar materials and exhibit adhesive properties. Polyphenols are natural products contained in plants and also the products we consume on a daily basis, such as tea and wine. Their famous examples are catechins and curcumin, which are extracted from tea leaves and turmeric, respectively (Fig. 1a, b). These polyphenols have a function of removing reactive oxygen species (ROS) and thus have various physiological activities such as effect to clean the skin and prevention of arteriosclerosis. In addition, polyphenols are characterized by their adhesive properties to biological tissues (astringent effect). The astringent effect is the phenomenon that polyphenols adhere to biological tissues such as the tongue via hydrophobic interactions and hydrogen bonds, and we feel bitterness when drinking wine or tea due to the adhesion (Fig. 1c).3 Thus, we construct a protein delivery system by utilizing their astringent effect on the nanoscale. Especially, tannic acid (TA, Fig. 1d), which is one of the polyphenols and consisting of 10 galloyl groups, is mixed with proteins in aqueous solution to form a stable protein/TA complex which protects the loaded-proteins. However, TA also interacts with blood vessel walls and biological components, which limiting the application as a drug delivery tool by itself.
 |
|||
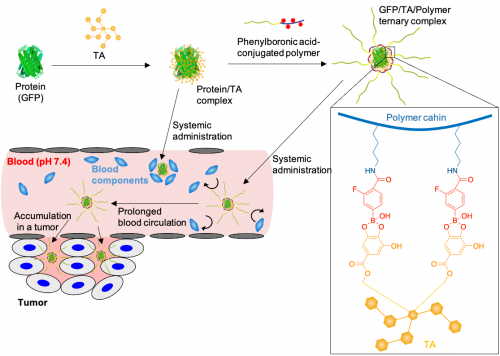
| Figure. 2 | Illustration of polyphenol-based nanomachine | ||
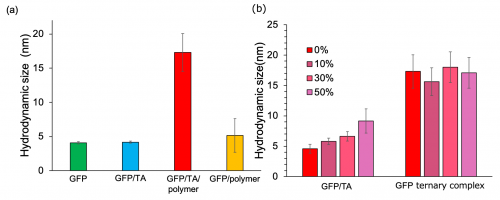
Therefore, we developed TA-based protein delivery system by combining a boronic acid-conjugated polymers that forms a boronate ester with TA (Fig. 2). Boronic acids could form a boronate ester in aqueous solution with a galloyl group of TA. Thus, the boronic acid-conjugated polymer could cover the protein/TA complex as shell, leading a construction of a core-shell type protein/TA/polymer ternary complex. The boronic acid-conjugated polymers were synthesized by conjugating multiple phenylboronic acids into the side chains of a biocompatible polymer, poly(ethylene glycol)-poly(L-Lysine). The synthesized boronic acid-conjugated polymers was mixed with protein/TA complex in aqueous solution, leading to significantly increase the particle size of the protein complex, and construct protein/TA/boronic acid-conjugated polymer ternary complex in aqueous solution by sequential self-assembly (Fig. 3a). Furthermore, detailed structural analysis revealed that the ternary complex forms a single molecule protein-loaded complex. Next, we evaluated the interaction of the prepared complexes with biological components (fetal bovine serum, FBS, Fig. 3b). The protein/TA complex exhibited increased hydrodynamic diameters with an increased concentration of FBS, suggesting that serum components interacted with the protein/TA complex. In contrast, the ternary complex maintained its size even in the presence of 50% FBS. The PEG shell might inhibit the interaction with biological components.
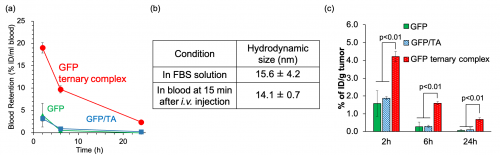
Finally, the prepared protein samples were intravenously injected into mice to evaluate their pharmacokinetics (Fig. 4). The intravenously injected protein ternary complexes showed significantly prolonged blood retention compared to the protein alone and the protein/TA complexes. This result indicated that protein ternary complexes could form stably even under the harsh in vivo condition. In fact, the particle size of the protein ternary complexes after administration was comparable to the particle size of the prepared ternary complexes in tube. Due to the stable complex formation and the prolonged blood retention, the tumor accumulation of the ternary complexes was also greatly improved compared to the protein alone.
 |
|||
| Figure. 4 | Biodistribution. (a) Blood retention after intravenous injection. (b) The stability of protein ternary complexes in in vitro and in vivo. (c) Tumor accumulation. | ||
These results indicate that tannic acid and boronic acid-conjugated polymers can form a self-assembled core-shell complex with proteins in aqueous solution, and the ternary complex functioned as a protein delivery system. Furthermore, the complexes showed dissociation properties in response to cellular endosomal pH (~5.5) and intracellular molecules (ATP). These properties indicates that the ternary complex not only delivers proteins to the disease site, but may also release the loaded-protein after accumulation in the cells of the site of disease. In addition, this ternary complex can load not only proteins but also a variety of molecules such as gene vectors and inorganic particles and therefore be expected to be further applied in the future.
This study was published in Biomacromolecules, a journal of the American Chemical Society in 2020 (Fig. 5)2.
 |
|||
| Figure. 5 | Cover picture showing the accumulation of protein ternary complexes in a tumor. | ||
Reference
| 1. | Pisal, D. S.; Kosloski, M. P.; Balu-Iyer, S. V. Delivery of Therapeutic Proteins. J. Pharm. Sci. 2010, 99 (6), 2557−2575. |
| 2. | Honda, Y.; Nomoto, T; Matsui, M.; Takemoto, H.; Kaihara, Y.; Miura, Y.; Nishiyama. N. Sequential Self-Assembly Using Tannic Acid and Phenylboronic Acid-Modified Copolymers for Potential Protein Delivery. Biomacro. 2020, 21 (9), 3826-3835. |
| 3. | Ma, S.; Lee, H.; Liang, Y.; Zhou, F. Astringent Mouthfeel as a Consequence of Lubrication Failure, Angew. Chem. Int. Ed. 2016, 55, 5793 -5797 |