Latest Research
- 2021.10.29
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
- Fujii Group
Chiral discrimination between tyrosine and beta-cyclodextrin revealed by cryogenic ion trap infrared spectroscopy
Keisuke Hirataa, c,Shun-ichi Ishiuchia, c,Anne Zehnacker-Rentienb, c and Masaaki Fujiic
a) Dep. Chem., Schl. Science, Tokyo Tech, b)U. Paris-Saclay, c) WRHI, IIR
In this article, I would like to introduce our joint research with Prof. Anne Zehnacker-Rentien in CNRS / U. Paris-Saclay, Profs Keisuke Hirata and Shun-ichi Ishiuchi in School of Science.
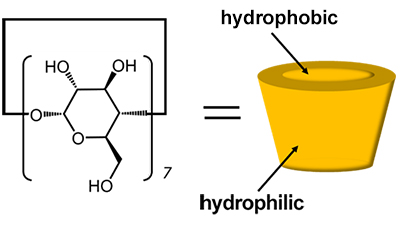
Cyclodextrins (CDs) are cyclic oligosaccharides composed of D-glucose linked by a-1,4 glycosidic linkages, which form vacancies in the molecule (Fig. 1). Since CD has chirality, when a guest molecule has chirality, each enantiomer has a different affinity for CD. CD has been used as a stationary phase in liquid chromatography and has been put to practical use as a chiral column for splitting optical isomers [1]. Although β-CD, which is composed of seven D-glucose moieties, is widely adapted to small guest molecules such as amino acids, the pore size of β-CD is 6 Å, which is smaller than that of amino acids (2~3 Å). However, the pore size of β-CD is 6 Å, which is much larger than the size of amino acids (2~3 Å), and it is not clear why such a large host can recognize the chirality of small guests.
 |
|||
| Fig. 1 Structure of b-CD | |||
In this study, we investigated the interaction between two enantiomers of protonated tyrosine and methyl β-CD (β-MCD), in which all OH groups of β-CD are methoxylated, by using cryogenic ion-trap infrared spectroscopy. We attempted to elucidate the mechanism of chiral discrimination of CD from the difference of the host-guest interaction. β-MCD eliminates the complex OH stretching vibrational bands derived from CD, and the vibrational bands derived from protonated tyrosine can be clearly observed.
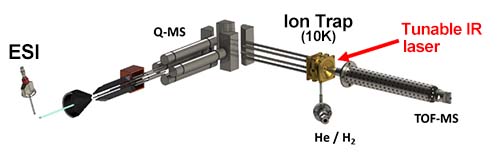
The experimental setup is shown in Fig. 2 [2-3]. The complexes of tyrosine and β-MCD, protonated using electrospray (ESI) method, were extracted into the gas phase, selected for specific masses using a quadrupole mass spectrometer, and trapped in a cooled ion trap (~10 K). Hydrogen gas was introduced to the complex ion, and molecular hydrogen was attached to the complex ion. When the complex ion was irradiated with a tunable infrared (IR) laser light, the molecular hydrogen dissociated by vibrational excitation due to IR absorption. The fragment ions (complex ions) generated by this dissociation were detected by a time-of-flight mass spectrometer (TOF-MS). By scanning the wavelength of the IR light while monitoring the amount of fragment ions, the IR photodissociation (IRPD) spectrum corresponding to the IR absorption spectrum was measured.
 |
|||
| Fig. 2 Experimental Setup | |||
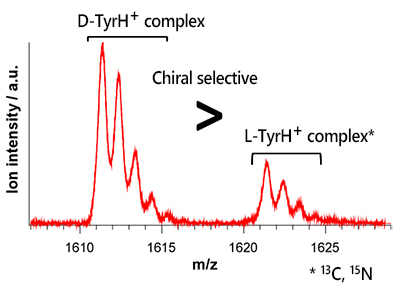
First, to investigate whether β-MCD is more selective for L- or D- tyrosine, we electrosprayed a solution containing equal amounts of L-tyrosine and D-tyrosine with β-MCD and measured the mass spectrum. Fig. 3 shows the mass spectrum of the complexes of protonated tyrosines and β-MCD, where the molecular weight of L-tyrosine is 10 times larger than that of D-tyrosine due to the isotope substitution (13C, 15N). The D-TyrH+ complex is three times stronger than the L-TyrH+ complex, indicating that β-MCD has a chiral selectivity for D-TyrH+.
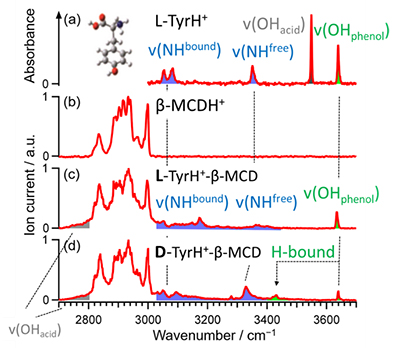
The IRPD spectra of each of the TyrH+ complexes were then measured in order to determine the structural basis for this difference in chiral selectivity. The IRPD spectra of the complexes of protonated tyrosine and β-MCD are shown in Fig. 4(c) and (d). From comparison with the spectra of tyrosine and β-MCD alone (Fig. 4(a) and (b)), bands at 3640 cm-1, 3430 cm-1, 3300~3400 cm-1, 3020~3300 cm-1 and 2700~2800 cm-1 are assigned to free phenol OH stretching without hydrogen-bonding, hydrogen-bonded phenol OH stretching, free NH stretching, hydrogen-bonded NH stretching and hydrogen-bonded carboxylic acid OH stretching vibration, respectively. As can be seen in Fig. 4, the "amino-bonded" form, in which the carboxylic acid OH and amino group NH of tyrosine form hydrogen-bonds with b-MCD, was observed in both complexes. On the other hand, in the D-TyrH+ complex, a band of hydrogen-bonded phenol OH was observed at 3430 cm‑1, suggesting that the "phenol-bonded" type, in which β-MCD and phenol OH are hydrogen-bonded, also coexists in addition to "amino-bonded" complex. These results are in agreement with the theoretical infrared spectra obtained by quantum chemical calculations.
 |
|||
| Fig. 3 Mass spectrum of complexes of b-MCD with L- and D-TyrH+ | |||
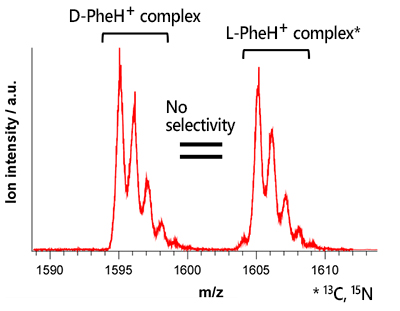
From these results, we conclude that the high chiral selectivity of β-MCD for D-TyrH+ is due to the fact that only the D-TyrH+ complex can take the phenolic bonded form. If this is the case, the chiral selectivity should be decreased without phenol OH. To confirm this, we measured the mass spectrum of β-MCD with phenylalanine (PheH+), in which the phenol OH of tyrosine is replaced by hydrogen (Fig. 5). As a result, unlike TyrH+, the D- and L-PheH+ complexes were observed with almost the same intensity, showing that the chiral selectivity of β-MCD for D-, L-PheH+ was lost. Thus, it was experimentally demonstrated that the key to the chiral selection of -MCD for tyrosine is whether or not the phenol-bound form can be prepared.
 |
|
| Fig. 4 | IR-UV dip spectra of (a) isomer A of L-TyrH+, (b) b-MCDH+,(c) L- and (d) D-TyrH+ with b-MCD complexes |
 |
|||
| Fig. 5 Mass spectrum of b-MCD with L- and D-PheH+ | |||
【References】
| [1] | D. W. Armstrong and W. Demond, J. Chromatogr. Sci., 1984, 22, 411., |
| [2] | S. Ishiuchi, et al., J. Mol. Spectrosc., 2017, 332, 45., |
| [3] | S. Ishiuchi, et al., Phys. Chem. Chem. Phys., 2019, 21, 561. |




