Latest Research
- 2021.10.01
Manipulation of Electron and Hole Transport in Organometallic Molecular Wires
In silicon-based semiconductors, there are n-type semiconductors that transport electrons and p-type semiconductors that transport holes. These are incorporated into integrated circuits used in smartphones and computers. On the other hand, molecular structure of organic materials determines conduction carriers (electrons or holes), and if these carriers can be manipulated as one wishes, they are expected to be applied as materials for molecule-based electronic circuits (molecular devices). In general, the conduction carrier of a molecule depends greatly on its skeleton and the terminal anchor groups [1]. In this study, we have developed a molecule whose conduction carrier can be easily controlled by simply modifying a part of the molecule (Fig. 1). This work was performed in collaboration with Dr. Swarup Chattopadhyay at University of Kalyani (India) and Ms. Shiori Ogawa of our laboratory [2].
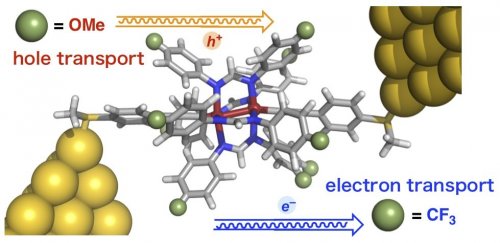 |
|||
| Figure 1: | Molecular junction of an organometallic molecular wire with controllable carrier transport process | ||
Our laboratory has previously reported that organometallic molecular wires with metal-carbon bonds exhibit high single-molecule conductance [3,4], and Dr. Chattopadhyay has extensively studied multinuclear complexes with metal-metal bonds[5]. Thus, we have designed and synthesized new paddlewheel dinuclear ruthenium molecular wires (Fig. 2). The name "paddlewheel" comes from the structure of the amidinato ligand, which connects the two rutheniums, being similar to the outer wheel of an ocean-going ship. We evaluated the conduction properties of the amidinato ligands with various substituents. Thiomethyl groups were also introduced at the end of the molecules for connection with gold electrodes.
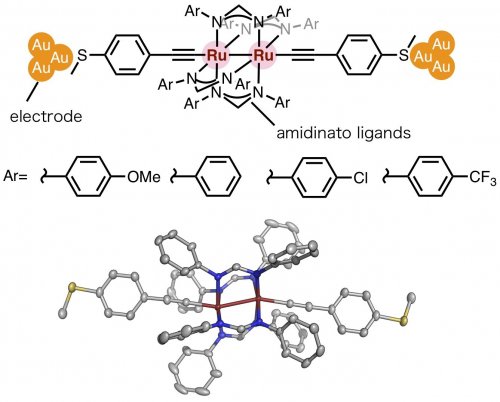 |
|||
| Figure 2: | (Top) molecular junction structure. (Bottom) Crystal structure of a paddlewheel-type dinuclear ruthenium molecular wires. | ||
Single-molecule conductance studies were performed by the STM break-junction method. Plots of the Hammett parameter, which is an indicator of the electron-donating and electron-withdrawing properties of substituents, versus the conductance show a U-shaped trend with a large conductance for compounds with electron-donating OMe substituents and electron-withdrawing CF3 substituents. This trend is quite different from the monotonic increase and decrease expected for n- and p-type molecules. In order to clarify the reason for this, the conduction orbitals were estimated by computations. As a result, it was found that the OMe derivative is HOMO conductance (p-type = hole transport), whereas the CF3 derivative is LUMO conductance (n-type = electron transport). This study is the first example of controlling the conduction carrier by simply changing the substituent of molecules.
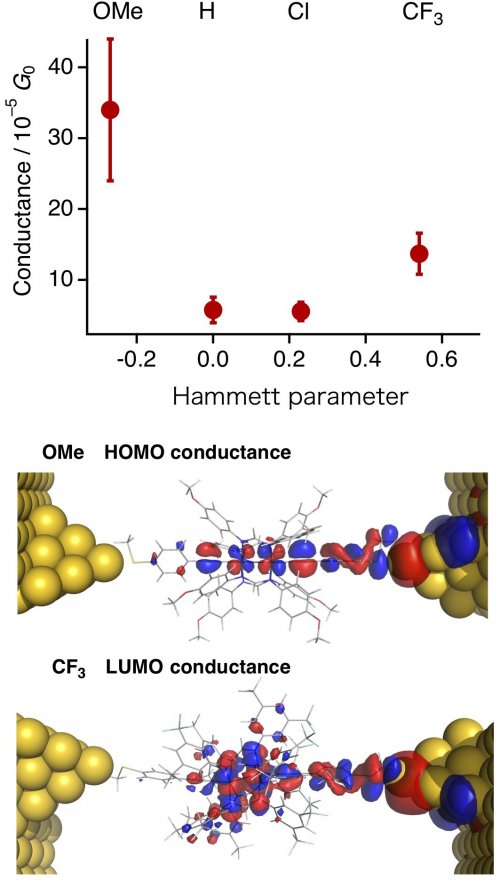 |
|||
| Figure 3: | (Top) Correlation between single-molecule conductance and Hammett parameter, and (Bottom) Conduction orbitals of the OMe and CF3 derivatives. | ||
To verify the reason for this phenomenon, we performed electrochemical and spectroscopic measurements and found that the HOMO-LUMO difference is about 1 eV, which is extremely small compared to common organic molecules. This indicates that both the HOMO and LUMO energies are located close to the Fermi level of the electrode (EF). Thus, the HOMO is close to EF for the OMe substituent and the LUMO is close to EF for the CF3 substituent (Fig. 4). This narrow HOMO-LUMO difference originates from the metal-metal bond, and we believe this is the reason for the unique substituent effect.
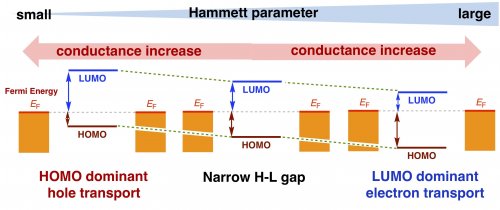 |
|||
| Figure 4: | Mechanism of substituent-induced change in conduction orbitals | ||
In this study, we have developed a molecule whose conduction carrier can be easily controlled by substituents. Molecules with controllable conduction carriers are expected to be applied not only to molecular devices but also to various appplications such as thermoelectric materials. In the future, we will continue our research to develop molecules that exhibit higher conductance and good thermoelectric properties.
| References | |
| [1] | S. Park, H. Kang, H. J. Yoon, J. Mater. Chem. A, 2019, 7, 14419-14446. |
| [2] | S. Ogawa, S. Chattopadhyay, Y. Tanaka, T. Ohto, T. Tada, H. Tada, S. Fujii, T. Nishino, M. Akita, Chem. Sci. 2021, 12, 10871-10877. |
| [3] | Y. Tanaka, Y. Kato, T. Tada, S. Fujii, M. Kiguchi, M. Akita, J. Am. Chem. Soc., 2018, 140, 10080-10084. |
| [4] | Y. Tanaka, Y. Kato, K Sugimoto., R. Kawano, T. Tada, S. Fujii, M. Kiguchi, M. Akita Chem. Sci. 2021, 12, 4338-4344. |
| [5] | S. Mallick, M. K. Ghosh, S. Mandal, V. Rane, R. Kadam, A. Chatterjee, A. Bhattacharyya, S. Chattopadhyay, Dalton Trans. 2017, 46, 5670-5679. |



