Latest Research
- 2021.11.01
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
- Hisabori-Wakabayashi Group
The regulation mechanism of ATP synthesis and hydrolysis unique to photosynthetic organisms.
Backgrounds
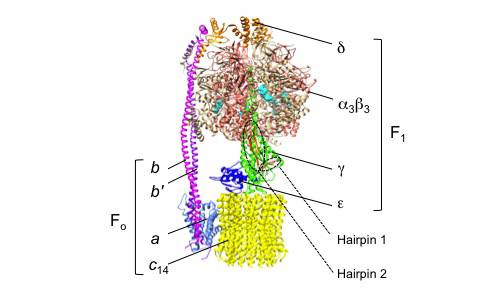
Photosynthetic organisms utilize FoF1 ATP synthase (FoF1) for solar-to-chemical energy conversion to produce ATP, the universal energy currency for cells. In the light, the photosynthetic electron transport is activated, generating a proton electrochemical gradient (proton motive force, pmf) across the thylakoid membrane, which drives ATP synthesis. In the dark, or when the pmf is insufficient, FoF1 hydrolyzes ATP as the reverse reaction and transports H+ to the lumen. FoF1 has a dual rotary-motor architecture, which couples the catalytic ATP synthesis/hydrolysis reactions with the transport of H+ across the biological membrane. FoF1 consists of a hydrophilic rotary motor moiety, F1, and an intramembrane rotary motor moiety, Fo (Fig. 1) [1]. F1 consists of five types of subunits with the stoichiometry of α3β3γδε, and the membrane-embedded Fo consists of three types of subunits with the stoichiometry of abb′cn, where 'n' is an integer between 8 (for mitochondria) to 14 (for chloroplasts and cyanobacteria) or 15 (for some cyanobacteria) depending on the origin of FoF1. The rotor shaft moiety is composed of the γε subunits, and the rotor-ring is composed of multimeric c subunits (c-ring). This rotor shaft rotates against the stator subunits, α3β3 of F1 and abb′ of Fo.
 |
|
| Figure 1 | A schematic diagram of FoF1 from Spinacia oleracea (PDB ID: 6FKF). |
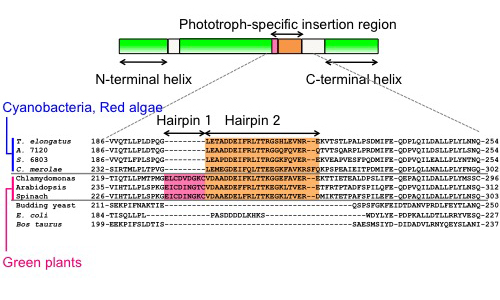
The γ subunit, a part of the rotor shaft of the F1 motor, comprises N- and C-terminal helical domains, as well as a protruding globular Rossmann fold domain located between these two helical parts (Fig. 2). The N- and C-terminal helical domains assemble into an antiparallel coiled-coil stalk, which is almost embedded in the central cavity of the catalytic headpiece, α3β3. The cyanobacterial and chloroplast γ subunits possess an inserted sequence of 30 or 39 amino acid residues within the Rossmann fold domain, forming a β-hairpin structure [1, 2, 3]. We previously demonstrated, by using recombinant α3β3γ subcomplexes, that this region plays an essential role in controlling the ATP hydrolysis activity [4, 5]. Furthermore, because the β-hairpin region-truncated α3β3γ exhibited higher ATP hydrolysis activity than the wildtype form, this region is thought to play an important role in regulating intracellular ATP levels in response to changes in the light environment through the control of ATPase activity. On the other hand, the role of this region in ATP synthesis has long remained an open question because its analysis requires the preparation of the whole FoF1 complex and a transmembrane proton-motive force.
 |
|
| Figure 2 | Comparison of the γ subunits from various organisms. Upper: The domain architecture of the γ subunit from the phototrophs. They possess a unique insertion sequence within the Rossmann-fold domain, between the N-terminal and C-terminal α helices. Lower: A partial alignment of amino acid sequences around the insertion region. The alignment was generated using the Clustal W multiple alignment tool. Cyanobacteria and red algae have phototroph-specific insertion, which forms a β-hairpin structure (Hairpin 2). Green plants and green algae have an additional β-hairpin structure (Hairpin 1). T. elongatus, Thermosynechococcus elongatus BP-1; A. 7120, Anabaena sp. PCC 7120; S. 6803, Synechocystis sp. PCC 6803; C. merolae, Cyanidioschyzon merolae. |
Here, we successfully prepared proteoliposomes containing the entire FoF1 ATP synthase from a cyanobacterium, Synechocystis sp. PCC 6803 (S. 6803), and measured ATP synthesis/hydrolysis and proton-translocating activities. The relatively simple genetic manipulation of S. 6803 enabled the biochemical investigation of the role of the β-hairpin structure of FoF1 ATP synthase and its activities. Our results indicated that this structure critically contributes to ATP synthesis and suppresses ATP hydrolysis [6].
Results
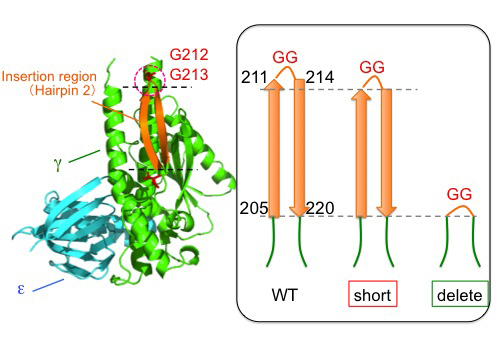
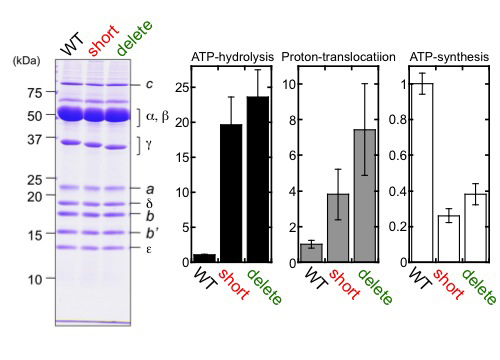
First, we genetically fused the His10-tag sequence to the N-terminus of the β subunit in S. 6803. The resulting S. 6803 was termed WT strain in the present study. Then, thylakoid membranes were prepared from the WT strain and solubilized, and the FoF1 preparation was purified from the solubilized fraction by Ni-affinity chromatography. Next, we prepared two β-hairpin structure-truncated strains of S. 6803, 'short' and 'delete' (Fig. 3), and purified the mutant FoF1 using the same procedure as described. No significant differences were observed in their subunit stoichiometries (Fig. 4).
 |
|
| Figure 3 | Construction of the β-hairpin-truncated mutants of S. 6803. Two amino acids located at the turn of the β-hairpin structure were truncated, along with the R211G and K214G substitutions, to maintain the β-hairpin structure in 'short' mutant. In contrast, the whole β-hairpin structure was truncated in 'delete' mutant. |
 |
|
| Figure 4 | Purification of FoF1 ATP synthase and analyses of ATP-hydrolysis, proton-translocating, and ATP-synthesis activity of reconstituted proteoliposomes. Each activity is shown as a ratio value to WT. |
The obtained FoF1 preparations were, respectively, reconstituted into soybean liposomes to obtain FoF1 proteoliposomes. Fig. 4 shows the relative ATP-hydrolysis, proton-translocating, and ATP-synthesis activities of the proteoliposomes. The results indicated that ATP-synthesis activity was lower, while ATP-hydrolysis and proton-translocating activities were higher in both mutants, compared to WT.
Discussions
Here, we established a single-step method for the preparation of S. 6803 FoF1. We then investigated for the first time the role of the phototroph-specific β-hairpin structure region of FoF1 in the ATP synthesis/hydrolysis activity of the enzyme. We demonstrated that ATP-synthesis was suppressed, and ATP-hydrolysis and proton-translocation were accelerated in the β-hairpin-truncated mutants. Overall, the results presented here indicate that the β-hairpin region of the γ subunit of FoF1 from S. 6803 critically contributes to its ATP synthesis activity and suppresses ATP hydrolysis.
We also performed physiological analyses using S. 6803 and found that the intracellular ATP content was significantly decreased in the β-hairpin-truncated mutants. However, under our experimental conditions, there was no remarkable effect other than the decrease in ATP, indicating the plasticity at the cellular level. Future research should elucidate the physiological function of the β-hairpin structure and its evolutionary significance for phototrophic organisms.
References
| [1] | Hahn, A., Vonck, J., Mills, D. J., Meier, T., and Kühlbrandt, W. (2018) Structure, mechanism, and regulation of the chloroplast ATP synthase. Science 360:eaat4318. |
| [2] | Murakami, S., Kondo, K., Katayama, S., Hara, S., Sunamura, E. I., Yamashita, E., Groth, G., and Hisabori, T. (2018). Structure of the γ-ε complex of cyanobacterial F1-ATPase reveals a suppression mechanism of the γ subunit on ATP hydrolysis in phototrophs. Biochem. J. 475:2925-2939. |
| [3] | Yang, J. H., Williams, D., Kandiah, E., Fromme, P., and Chiu, P. L. (2020) Structural basis of redox modulation on chloroplast ATP synthase. Commun. Biol. 3:482. |
| [4] | Konno, H., Murakami-Fuse, T., Fujii, F., Koyama, F., Ueoka-Nakanishi, H., Pack, C. G., Kinjo, M., and Hisabori, T. (2006) The regulator of the F1 motor. Inhibition of rotation of cyanobacterial F1-ATPase by the ε subunit. EMBO J. 25:4596-4604. |
| [5] | Sunamura, E. I., Konno, H., Imashimizu-Kobayashi, M., Sugano, Y., and Hisabori, T. (2010) Physiological impact of intrinsic ADP inhibition of cyanobacterial FoF1 conferred by the inherent sequence inserted into the γ subunit. Plant Cell Physiol. 51:855-865. |
| [6] | Kondo, K., Izumi, M., Inabe, K., Yoshida, K., Imashimizu, M., Suzuki, T., and Hisabori, T. (2021). The phototroph-specific β-hairpin structure of the γ subunit of FoF1-ATP synthase is important for efficient ATP synthesis of cyanobacteria. J. Biol. Chem. 475:2925-2939. |



