Latest Research
- 2022.01.13
- Fukushima-Shoji Group
A diborane(4) derivative exhibiting dual fluorescence and solvent-viscosity sensing capability arising from the B-B bond rotation
Neutral three-coordinate boranes exhibit strong Lewis acidity and electron-accepting properties due to the presence of a vacant 2p orbital of the boron atom (Fig. 1a). We have been working on the development of new boron species that can make full use of the properties of the vacant orbitals of boron. Examples of boron compounds we have developed so far include a diarylborinium ion (Mes2B+), a cationic boron compound in which the boron atom has only two chemical bonding (Fig. 1a,b)[1]. We have found that this borinium ion exhibits extraordinary reactivity. For example, Mes2B+ can cleave a stable C=O double bond of CO2 at room temperature. The unusual electron-deficient structure of Mes2B with two vacant 2p orbitals on the boron atom is responsible for such interesting reactivity.
 |
|
| Fig. 1. |
(a) Schematic illustrations of boron species. The lobes on the boron atoms represent vacant 2p orbitals. (b) Chemical structure of diarylborinium ion Mes2B+.
|
Diborane(4) with a boron-boron bond may show interesting properties arising from the neighboring vacant 2p orbitals of boron (Fig. 1a). However, as represented by bis(pinacolato)diboron, most of the previously reported diborane(4) derivatives are electronically stabilized by lone pair-donating heteroatom substituents on the boron atoms. While several examples of tetraaryldiborane(4) derivatives without heteroatom substituents have been reported[2], they are easily decomposed by hydrolysis. In order to make full use of the properties of two neighboring vacant 2p orbitals of diborane(4), a molecular design that can realize chemical stability without using lone pair-donating heteroatom substituents is desirable.
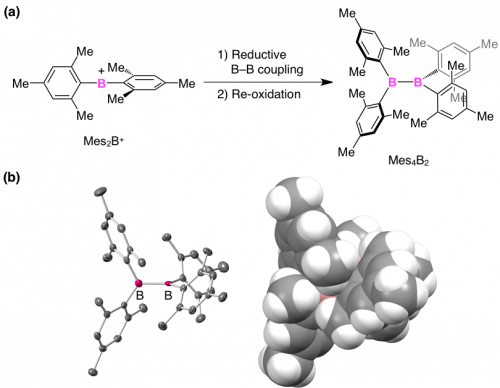
We found that above-mentioned Mes2B+ provides a solution to this issue. Upon treating with a Li metal, Mes2B+ underwent reductive boron-boron coupling, which enabled the synthesis of tetraaryldiborane(4) Mes4B2 (Fig. 2a)[3]. Single-crystal X-ray analysis of Mes4B2 shows that the B-B center is almost completely hidden by the four mesityl (2,4,6-trimethylphenyl) groups (Fig. 2b). Notably, Mes4B2 shows exceptional chemical stability; it is not hydrolyzed even in water. This excellent chemical stability allowed us to investigate the properties of Mes4B2 in detail.
 |
|
| Fig. 2. |
(a) Synthesis of tetraaryldiborane(4) Mes4B2 by the reductive coupling of borinium ion Mes2B+. (b) Crystal structure of Mes4B2 (left: thermal ellipsoid, right: CPK description).
|
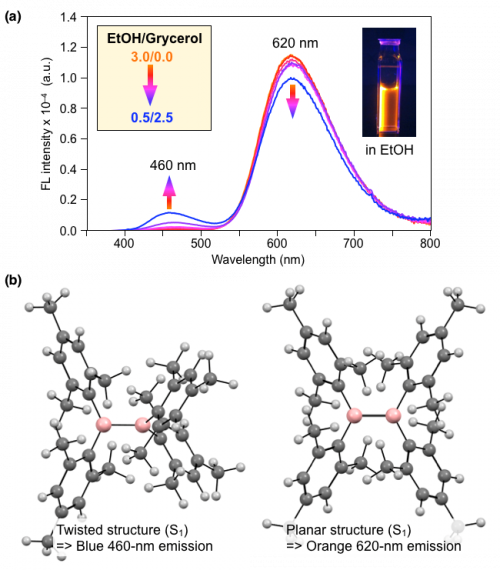
Interestingly, Mes4B2 shows a bright orange fluorescence, which was unexpected for such a simple molecule. We found that the observed orange emission includes a low-intensity shorter-wavelength band (λmax = 460 nm) and high-intensity longer-wavelength band (λmax = 620 nm) (Fig. 3a). Thus, Mes4B2 exhibits dual fluorescence.
Fig. 3. (a) Fluorescence spectra of Mes4B2 in in ethanol/glycerol (3.0/0.0 to 0.5/2.5 v/v) at 25 °C (Λex = 360 nm).(b). Calculated equilibrium structures of Mes4B2 in the excited S1 state.
To understand the emission behavior of Mes4B2, we carried out theoretical calculations on the conformation and energy of Mes4B2 in the ground (S0) and excited (S1) states. We found that (1) in both the S0 and S1 states, the rotation of the boron-boron bond can take place with a low activation barrier, and (2) there are two equilibrium structures (twisted and planar structures) in the S1 state (Fig. 3b). Importantly, the shorter-wavelength (460 nm) and longer-wavelength (620 nm) emissions are attributed to the twisted and planar structure in the S1 states, respectively.
As expected from this calculation result, the relative intensity of the dual fluorescence is greatly affected by the viscosity of solvent. When a high-viscosity glycerol is mixed with ethanol, the relative intensity of the shorter-wavelength emission (λmax = 460 nm, derived from the twisted S1 structure) became increased (Fig. 3a). This is most likely due to the suppression of the rotational dynamics of the boron-boron bond in high-viscosity media. Therefore, Mes4B2 can serve as a ratiometric viscosity sensor.
The solid-state environment also affects the dual fluorescence behavior of Mes4B2. Mes4B2 can be subjected to vacuum evaporation to form an amorphous thin film. When this amorphous film is annealed at 80 °C, it gradually crystallizes. When observing the emission of the thin film in the annealing process, while the initial amorphous thin film shows orange emission (λmax = 620 nm, derived from the planar S1 structure), the crystallized film shows blue emission (λmax = 460 nm, derived from twisted S1 structure) (Fig. 4). This is because the rotational dynamics of the boron-boron bond are allowed in the amorphous state, whereas they are suppressed in the crystalline state.
In summary, we have revealed the interesting dual fluorescence properties of Mes4B2, derived from its rotational dynamics of the boron-boron bond. We are currently investigating peculiar chemical reactivity of Mes4B2 arising from the neighboring vacant 2p orbitals of boron.
In addition to diborane(4), various researches on the boron chemistry are ongoing in our group. Our recent publications include (1) the synthesis of boron-containing macrocycles exhibiting a strong borane-olefin proximity interaction (Fig. 5a)[4], (2) unique emission properties of a B2N2-heterocyclobutadiene derivative in which C=C bonds are replaced with B-N bonds (Fig. 5b)[5], and (3) the synthesis of a B4N4-heteropentalene derivative and its application to OLED (Fig. 5c)[6].
Fig 5. (a) Boron-containing macrocycle exhibiting a strong borane-olefin proximity interaction[4]. (b) B2N2-heterocyclobutadiene emitting blue phosphorescence in solution at room temperature[5]. (c) B4N4-heteropentalene that can serve as a host material for OLED[6].
|
References
|