Latest Research
- 2022.02.01
- Nakamura-Okada Group
Palladium-Catalyzed N-H/B-H Double Functionalization of 1,2-Azaborines
Heterocycles containing boron and nitrogen atoms in their ring systems, the so-called BN heterocycles, have attracted immense interest in various research fields including material science and medicinal chemistry.1 Among those BN heterocycles, much attention has been paid to azaborines whose structure is isoelectronic to benzene (Fig. 1). To expand their complexity for development of novel BN heterocycle-based materials, synthetic methods to provide multifunctionalized BN heterocycles in a highly enantioselective manner are required.
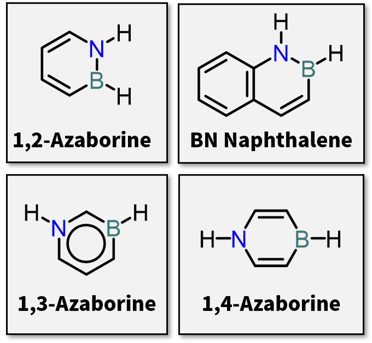 |
| Fig.1 Structure of azaborines and BN naphthalene |
In this context, direct functionalization of these compounds, especially 1,2-azaborines has been investigated in the last decade.2 However, current methods for N-H or B-H substitution require stoichiometric amount of strong bases, which resulted in limited structural diversity of these hetero atoms. Therefore, it is necessary to develop mild and catalytic N-H or B-H functionalization methods, though there is only one report that demonstrated rhodium-catalyzed B-H alkenylation.4
In this work, we have developed a palladium-catalyzed N-H/B-H double functionalization of 1,2-benzazaborines 1 via cycloaddition with vinylethylene carbonate (2) under mild conditions (Fig. 2). This reaction enabled the synthesis of a wide variety of novel polycyclic oxazaborolidines 3 with good functional group tolerance.
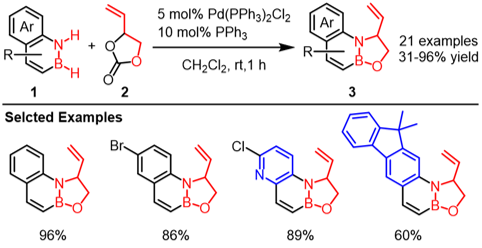 |
|
| Fig. 2 | Pd-catalyzed cycloaddition via N-H/B-H double functionalization of 1,2-azaborines |
Furthermore, we succeeded in asymmetric cycloaddition by using a chiral phosphoramidite ligand L1 to produce oxazaborolidine 3 with high enantioselectivity (Fig. 3). Further derivatization via transition-metal catalyzed transformation of the vinyl group in 3 proceeded smoothly to give various functionalized BN heterocycles with retention of chirality.
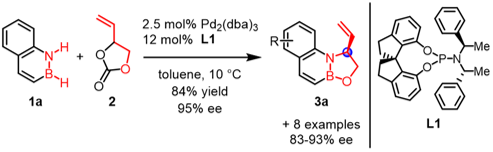 |
| Fig.3 Pd-catalyzed asymmetric cycloaddition of 1.2-azaborines |
In summary, mild, catalytic, and enantioselective N-H/B-H difunctionalization of 1,2-azaborines was successfully demonstrated. To the best of our knowledge, this is the first report on the asymmetric direct functionalization of BN heterocycles. Our synthetic methodology will facilitate the development of BN heterocycle-based bioactive molecules and materials. Further investigations on the direct functionalization of BN heterocycles are underway in our laboratory.
References
| 1. | Z. X. Giustra, S.-Y. Liu, J. Am. Chem. Soc. 2018, 140, 1184-1194. |
| 2. | C. R. McConnell, S.-Y. Liu, Chem. Soc. Rev. 2019, 48, 3436-3453. |
| 3. | A. N. Lamm, E. B. Garner III, D. A. Dixon, S.-Y. Liu, Angew. Chem. Int. Ed. 2011, 50, 8157-8160. |
| 4. | A. N. Brown, L. N. Zakharov, T. Mikulas, D. A. Dixon, S.-Y. Liu, Org. Lett. 2014, 16, 3340-3343. |
| 5. | T. Morita, H. Murakami, Y. Asawa, H. Nakamura, Angew. Chem. Int. Ed. early view (2022), DOI: 10.1002/anie.202113558. |



