Latest Research
- 2022.12.06
- Nishiyama-Miura Group
Cancerous pH-responsive Polycarboxybetaine-coated Nanoparticle for Smart Delivery of siRNA against Subcutaneous Tumor Model
Small interfering RNAs (siRNAs) are used for the treatment of multiple diseases, including cancer. Notably, siRNAs are susceptible to degradation inside cells due to their physiochemical properties, making their delivery difficult. Hence, lipid nanoparticles (LNPs) are generally used as carriers for the systemic delivery of siRNAs, due to their biocompatibility, low-toxicity, and ability to encapsulate siRNA.
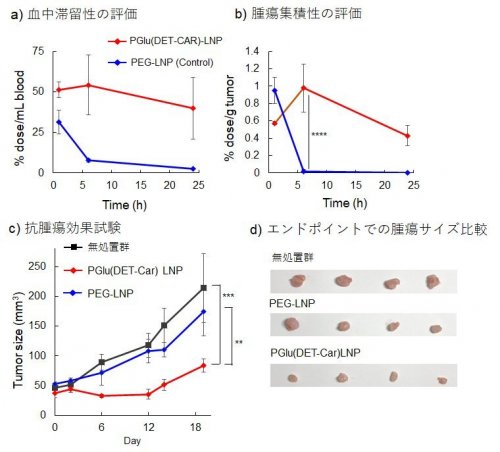
The external surfaces of LNPs play a key role in differentiating between a normal physiological environment and a cancerous environment. An ideal LNP system should be able to recognize tumor-specific conditions and deliver the required siRNA to the targeted tumor. Such LNPs typically consist of a pH-responsive lipid which identifies the tumor environment, and a polyethylene glycol (PEG) lipid, which encapsulates the siRNA and delivers it to the target site. At present, these LNPs lack the ability to deliver siRNAs to tissues and organs other than the liver. Recently, we designed an LNP through surface modifications which allow the molecule to remain neutrally charged at a physiological pH of 7.4, but switch to a positive or cationic charge upon sensing the acidic pH of cancerous tissues in its vicinity (Fig. 1). We hoped that the positive charge of the LNPs would allow them to target anionic cancerous tissues with high efficacy. To achieve this, they used an "ethylenediamine-based polycarboxybetain" molecule as a "smart shell" for the LNPs [1. 2], which demonstrated the ability to switch to cationic charge in response to acidic pH (Fig. 2). This polycarboxybetain-modified LNP could enhance cellular uptake in cancerous pH, resulting in facilitated endosomal escape and gene knockdown efficiency (Fig. 3). After systemic administration, the polycarboxybetaine-modified LNP could seamlessly circulate through the blood at physiological pH, while enhancing cellular uptake of siRNA in cancerous tissues and delivering required nucleic acids to solid tumors. In addition, it effectively targeted tumor cells in two in-vitro tumor models, leading to tumor growth inhibition (Fig. 4).
 |
|
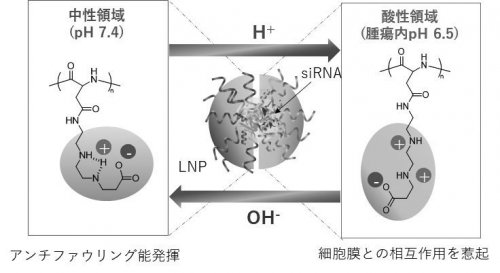
| Fig. 1 |
Structure of DSPE-PGlu(DET-Car) and functuons of siRNA-encapsulated PGlu(DET-Car) LNP.
|
 |
|
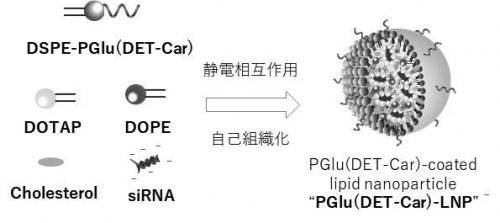
| Fig. 2 |
Schematic illustration of PGlu(DET-Car) LNP.
|
Overall, our study demonstrates the potential of pH-responsive polycarboxybetaine as a surface coating molecule for LNP, which can facilitate the effective and targeted delivery of nanomedicines like siRNAs. The polycarboxybetain-modifed LNP designed by us offers promise as a delivery vehicle in the treatment of cancers and other inflammatory diseases that are characterized by an acidic microenvironment.
 |
|
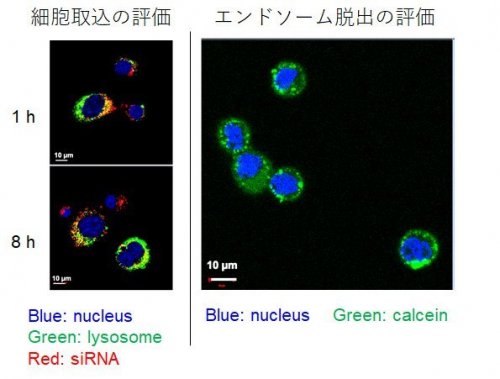
| Fig. 3 |
Assessment of cellular uptake and endosomal escape. CLSM image of 1 h and 8 h treatment of Alexa647-labeled siRNA-encapsulated PGlu(DET-Car)30 LNP in CT 26.
|
| 1. | Ranneh A -H, et al : Angew. Chem. Int. Ed. 57, 5057 (2018). |
| 2. | Awaad A, et al : J. Control Release. 346, 392 (2022). |
| 3. | Harada H, et al : Biol. Pharm. Bull., 36(6), 892 (2013). |