Latest Research
- 2023.02.01
- Yamamoto-Imaoka Group
Electron Microscopy Opens Up the Chemistry of Atoms and Their Assemblies
The essence of chemical bonding is the recombination of bonds between atoms. Simply put, the study of chemical bonds between typical elements or between transition metals and typical elements and their properties is called organic chemistry, coordination chemistry, and inorganic chemistry. Chemistry has been developed by isolating substances, i.e., stable molecules and solids, analyzing their structures, and evaluating their physical properties. On the other hand, the existence of many intermetallic bonds has never been confirmed. This is because many of the bonds are weaker than those of typical elements and cause phase separation, making it difficult to isolate them as molecules or solids. However, recent catalytic science has focused on the importance of not only stably isolated compounds but also sub-nanoscale metals and tiny atomic aggregates that are formed in a very short time. According to theoretical calculations, such atomic assemblies do not always have the same static structure but are thought to have a dynamic structure that undergoes isomerization very frequently. Therefore, X-ray structural analysis, which has been used in chemistry to determine the structure of molecules and solids, cannot resolve the substance because its structure is averaged over time. Unfortunately, the structure of sub-nanomaterials could only be described as "amorphous."
Electron microscopy is a very powerful tool as a method for exploring the nature of these not-always-stable substances; the innovation in biomolecular structure analysis sparked by cryo-electron microscopy, which won the 2017 Nobel Laureate in Chemistry, is still fresh in our minds. Due to recent advances in aberration correction techniques, electron microscopy is one of the tools that can directly observe the extremes of smaller chemical entities - a single atom - in real-time. More recently, atomic resolution observations have been realized at low acceleration voltages to avoid material destruction by accelerated electron beam probes, allowing direct observation of atom dynamics. However, many of the results reported in the late 2000s and early 2010s regarding the observation of metal atomic aggregates (clusters) were considered to be the process of particle decay rather than the steady state of matter.
We have directly observed for the first time the reversible and continuous structural change of a Pt4 cluster consisting of four platinum (Pt) atoms for more than 100 s [1]. Long-term continuous imaging at atomic resolution was achieved by setting the acceleration voltage of the probe to less than 80 kV and the current to less than 10 pA. This allowed the observation of reversible structural changes. Observation with the low-voltage and low-current probe has become a new way to visualize not only the structure of clusters consisting of a few atoms but also the direct observation of macromolecular metal complexes for which there was no way to study the structure[2]. The atomic arrangement structures found in Pt4 were classified into several types. It was found that in the steady state, the atomic arrangement structures reversibly isomerize back and forth between the finite number of structures at a constant rate. In other words, the structure that was originally called amorphous is "invisible" as a result of the time averaging of the structure. The term amorphous, meaning disordered structure, is not necessarily appropriate. In fact, there are many examples of subnanoparticles that exhibit catalytic activity that depends on the number of constituent atoms. Such properties cannot be explained by an unstructured amorphous form, but must be attributed to some "structure".
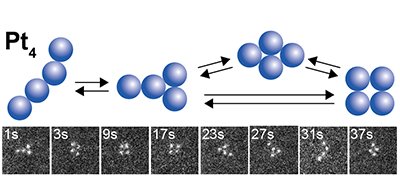 |
|
| Figure 1. |
Electron microscope images of Pt4 cluster and its structural isomerization.
|
As an example of the observation of slightly larger atomic assemblies, oxide and carbide subnanoparticles of the transition metal molybdenum (Mo) have been systematically prepared on a carbon support and their direct observation has been successful [3]. Continuous time course observations have also visualized the liquid-like dynamics of Mo atoms that exist only inside the subnanoparticles. Particles larger than 3 nm in diameter were found to form carbides such as β′-Mo2C, while smaller particles formed acid carbides without crystalline structure, confirming that the crystalline phase changes with composition in a size-dependent manner. This is the first demonstration of such size-dependent phase transformation at the ultramicroscopic scale.
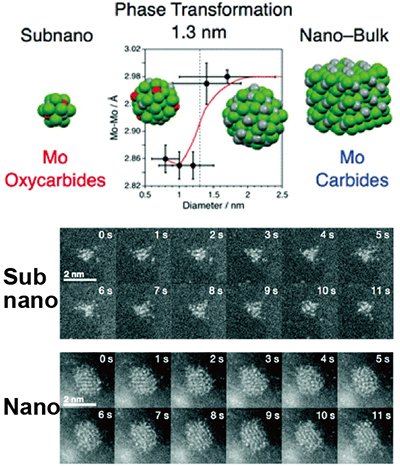 |
|
| Figure 2. | Differences in structure, composition, and atomic dynamics of molybdenum carbide at the nano- to sub-nanoscale (electron microscope image) |
On the other hand, the chemically stable superatomic subnanoparticles behave differently from ordinary subnanoparticles: Ga13, a superatomic material that has halogen-like properties in addition to Al13, retains a relatively stable spherical morphology under electron microscopic observation. The difference is striking in contrast to Pt subnanoparticles, whose outer shape also changes as the internal atoms move [4]. Thus, the observation of atom dynamics has proven to be useful in the search for advanced materials such as superatoms.
 |
|
| Figure 3. |
Electron microscope images of Ga13, Ga12 and Pt13 on graphene
|
If we can identify two or more elements and directly observe their atomic dynamics, including bond formation, we can find many substances that have never been discovered before. This requires elemental identification of each individual atom. Elemental analysis combined with electron microscopy is commonly performed by EDS (energy dispersive X-ray spectroscopy) and EELS (electron energy loss spectroscopy), but both have insufficient detection sensitivity for single atoms and are difficult to apply to moving atoms due to the beam damage caused by high-current electron irradiation. We have developed a high-precision elemental identification method based on the principle of correlation between the brightness of an atom and its atomic number in annular dark-field scanning transmission electron microscopy (ADF-STEM). This method takes full advantage of the sequential analysis of moving images. For the first time, the dynamics of formation and dissociation of dimers and trimers composed of different elements were directly visualized at atomic resolution, and the elements were identified[5]. The successful direct identification of dimers and trimers (molecules) composed of different atoms, such as AgAg, AgCu, and AuAgCu (Figure 4), was achieved by identifying moving atoms. Notably, the results show that even such metallic elements that are phase-separated in bulk, such as Ag-Cu and Au-Ag-Cu, can be mixed and bonded at the atomic level on the sub-nanoscale.
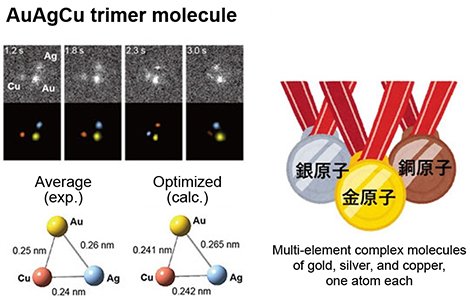 |
|
| Figure 4. |
Gold-silver-copper triatomic molecules were first discovered by electron microscopy.
|
Subnanoscale alloying is always effective for inter-elemental synergies in catalysis. Thirty-six combinations of bimetallic subnanoparticles (SNPs) and nanoparticles (NPs) have been systematically studied using catalytic benchmarks based on atomic resolution imaging and electrochemical hydrogen evolution reactions (HER). The results show that SNPs consistently produce greater synergistic effects than NPs (Figure 5). The greatest synergy was obtained with the combination of Pt and Zr [6]. It is noteworthy that the uniform atomic-level mixing of Pt and Zr occurs at the subnanoscale, even though Zr is oxidized to an oxide by atmospheric oxygen. The ability of advanced electron microscopy to reveal the previously invisible subnanoscale structure of materials is expected to accelerate the chemistry of atoms and their aggregates, leading to their application in catalysis and other fields.
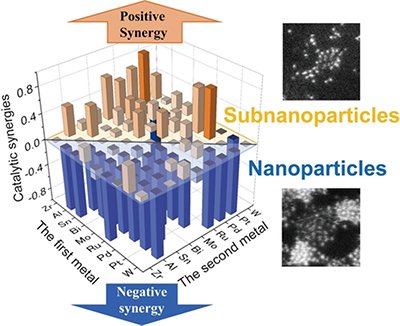 |
|
| Figure 5. | The synergistic effect between two elements in the hydrogen evolution reaction (HER) is stronger for subnanoparticles than for nanoparticles. |
| [1] | T. Imaoka, T. Toyonaga, M. Morita, N. Haruta, K. Yamamoto, Chem. Commun. 2019, 55, 4753-4756. |
| [2] | K. Takada, M. Morita, T. Imaoka, J. Kakinuma, K. Albrecht, K. Yamamoto, Sci. Adv. 2021, 7, eabd9887. |
| [3] | M. Wakizaka, A. Atqa, W.-J. Chun, T. Imaoka, K. Yamamoto, Nanoscale 2020, 12, 15814-15822. |
| [4] | T. Kambe, A. Watanabe, M. Li, T. Tsukamoto, T. Imaoka, K. Yamamoto, Adv. Mater. 2020, 32, 1907167. |
| [5] | M. Inazu, Y. Akada, T. Imaoka, Y. Hayashi, C. Takashima, H. Nakai, K. Yamamoto, Nature Commun. 2022, 13, 2968. |
| [6] | Q. Zou, Y. Akada, A. Kuzume, M. Yoshida, T. Imaoka, K. Yamamoto, Angew. Chem. Int. Ed. 2022, 61, e202209675. |



