Latest Research
- 2023.04.03
- Fukushima-Shoji Group
Construction of Zn-oxo clusters using rigid tridentate triptycene-based ligands
Ligated metal-ion clusters have attracted considerable attention due to the characteristic optoelectronic, magnetic, and multi-redox properties they can potentially exhibit. To develop a rational strategy for the synthesis of such ligated metal-ion clusters, understanding the processes leading to their formation is important. However, as is referred to as "serendipitous assembly", the synthesis of metal-ion clusters is often due to spontaneous assembly of metal ions and organic ligands. To gain insight into the mechanism of ligated metal-ion cluster formation, it would be helpful to trace the reaction pathways leading to the final products. If this could be achieved, one could address, whether the accumulation of metal ions causes the assembly of ligands or the assembly of ligands causes the accumulation of metal ions; the "chicken-and-egg" issue in ligated metal-ion cluster formation.
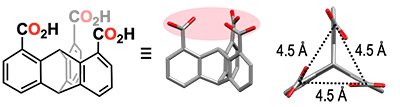
However, such attempts appear difficult, when using common flexible multidentate ligands having large degrees of freedom. This is because, the energy diagram leading to the final product is complex. Conversely, we imagined that the use of a rigid multidentate ligand having limited structural degrees of freedom might enable tracing of the metal-ion cluster formation processes. In the present study, we investigated the formation of Zn-oxo clusters using 1,8,13-tricarboxytriptycene (H3L) as a rigid tridentate ligand (Fig. 1).
 |
|
| Fig. 1. | Chemical structure of H3L and a schematic illustration of its three-dimensional structure. |
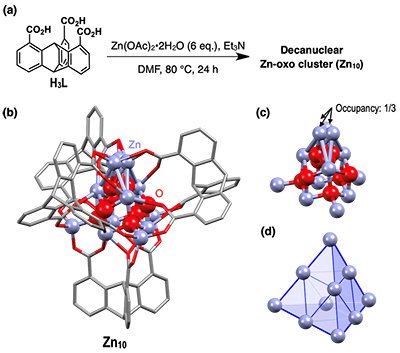
The complexation of 1,8,13-tricarboxytriptycene (H3L) with Zn(OAc)2•2H2O (6.0 eq.) was examined (Fig. 2a) in dimethylformamide. Negative-mode electrospray ionization mass spectrometry (ESI-MS) of the resulting solution displayed distinct molecular ion peaks, originating from zinc clusters composed of four triptycene ligands and ten Zn ions. After many trials, we obtained colorless hexagonal crystals which upon single-crystal X-ray analysis revealed the formation of a neutral decanuclear Zn-oxo cluster (Zn10) composed of [Zn10(µ4-O)4(µ3-H2O)3(L)4] (Fig. 2b). The Zn-oxo core has a tetrahedral shape with µ4-O bridges and features a tetragonal-shaped ZnO substructure (Fig. 2c,d).
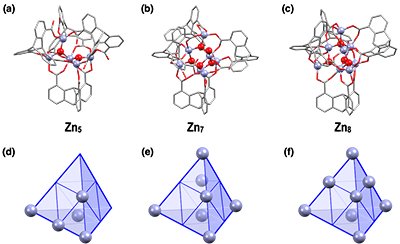
Depending on the feed ratio of H3L and Zn(OAc)2•2H2O, the distribution of Zn10 and other Zn-oxo clusters containing smaller numbers of Zn(II) varied. ESI-MS spectra of the products obtained from the reactions of H3L and Zn(OAc)2•2H2O at different molar ratios showed the molecular ion peaks attributed to clusters containing five to ten zinc ions per four triptycene ligands. The number of zinc ions in the clusters increased with equivalents of zinc acetate dihydrate and when the molar ratio was greater than 6.0, Zn10 was the sole product. Fortunately, we successfully obtained single-crystal salts of anionic penta-, hepta-, and octanuclear Zn-oxo clusters, Zn5, Zn7, and Zn8, respectively, suitable for X-ray crystallographic analysis (Fig. 3). Interestingly, the arrangements of the triptycene ligands in these Zn-oxo clusters are almost identical with one another, indicating that Zn5, Zn7, and Zn8 are considered to be intermediates of Zn10.
 |
|
| Fig. 3. |
X-ray crystal structures of the anionic moieties of (a) Zn5, (b) Zn7, and (c) Zn8. Schematic illustrations of the Zn(II) arrangements in (d) Zn5, (e) Zn7, and (f) Zn8.
|
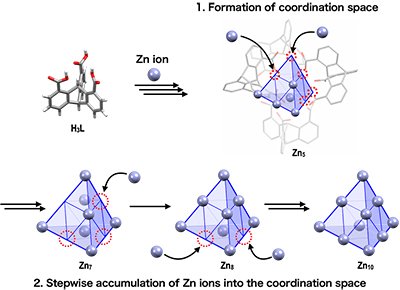
Therefore, the time course of the formation of decanuclear Zn-oxo clusters were traced by ESI-MS. As a result, it was found that pentanuclear clusters were formed immediately after the start of the reaction, clusters with a larger number of Zn ions appeared with the passage of time, finally converging to only decanuclear Zn-oxo clusters. These results reveal the cluster-formation process, where four triptycene ligands preorganize to form a robust coordination space to which Zn ions accumulate in a stepwise manner up to Zn10 (Fig. 4). Why does the reaction converge with the formation of Zn10? This is because, the coordination space of Zn10 is fully occupied, and thus, no more Zn ions can be accumulated.
 |
|
| Fig. 4. |
Schematic illustration of the stepwise accumulation of Zn(II) ions in the coordination space of a pentanuclear Zn-oxo cluster.
|
In this study, we have revealed the formation mechanism of Zn-oxo clusters using rigid tridentate triptycene ligands[1]. We have also been able to bring one insight into the "chicken-and-egg" problem in this system. Currently, we are working on the construction of metal-ion clusters exhibiting various electronic and magnetic properties using triptycene-based rigid multidentate ligands.。
| [1] | M. Kato, T. Fukui, H. Sato, Y. Shoji, T. Fukushima, Inorg. Chem. 2022, 8, 3649-3654. (https://doi.org/10.1021/acs.inorgchem.1c03758) |