Latest Research
- 2023.07.21
- Nakamura-Okada Group
Efficient Boron Neutron Capture Therapy for Brain Tumor with Novel Boron Carrier
Boron Neutron Capture Therapy (BNCT) has attracted attention as a cancer treatment based on nuclear reaction between boron-10 (10B) and thermal neutron. It is especially explored as a novel therapeutic approach for refractory malignant brain tumors, particularly malignant gliomas, which is difficult to be treated by conventional surgical and radiation therapies. Currently, 4-borono-L-phenylalanine (L-BPA) is the only clinically approved boron agent for BNCT. There is a need to develop boron agents that are efficiently taken up by glioma cells in order to achieve sufficient therapeutic effects. In this study, we have developed a boron agent called PBC-IP, which exhibits high uptake in glioblastoma cells known as a highly malignant and refractory glioma. The BNCT using PBC-IP demonstrated remarkable therapeutic effects in both glioma and glioblastoma animal models. Herein, we present the detailed findings of this study.
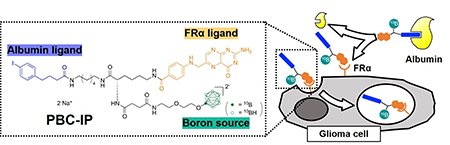
Previously, we had developed PBCs, which consist of a folate receptor ligand (pteroyl group) and dodecaborate containing 12 boron atoms, for targeting FRα highly expressed on glioma cells[1]. On the other hand, we successfully improved the blood retention and tumor accumulation of boron agents by conjugating dodecaborate with an iodophenyl group that binds to albumin in the blood, utilizing the enhanced permeability and retention (EPR) effect[2]. On the basis of these background, we have developed a novel compound called pteroyl-closo-dodecaborate-conjugated 4-(p-iodophenyl)butyric acid (PBC-IP), which incorporates the folate receptor α (FRα) ligand (pteroyl group), albumin-binding moiety (iodophenyl group), and boron source (dodecaborate) (Fig. 1)[3].
 |
|
| Fig. 1 | Design and cellular uptake mechanism of pteroyl-closo-dodecaborate-conjugated 4-(p-iodophenyl)butyric acid (PBC-IP). |
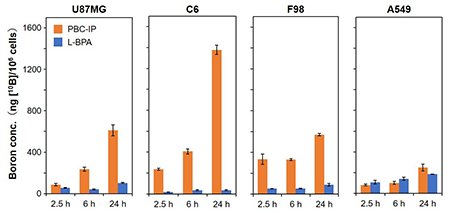
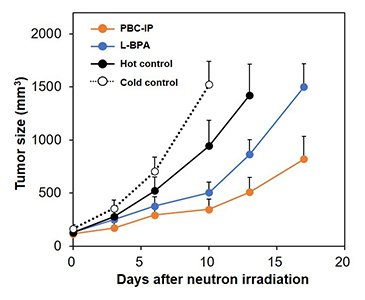
The dissociation constant (Kd) of PBC-IP with albumin was calculated to be 1.7 μM by ELISA, indicating higher affinity compared to BC-IP (Kd = 148.0 μM). The results suggested that the pteroyl group contributed to the interaction with albumin. Cellular uptake of PBC-IP was evaluated by using human and rat glioma cell lines, including U87MG (human glioblastoma), C6 (rat glioma), and F98 (rat glioma), demonstrating that the boron concentration in the cells exposed to PBC-IP was 10-20 times higher than L-BPA (Fig. 2). In contrast, the boron uptake in A549 cells (human lung cancer) was equivalent between PBC-IP and L-BPA, indicating that PBC-IP particularly tends to accumulate in the glioma cells. We performed the thermal neutron irradiation to U87MG xenograft mouse models intravenously injected with PBC-IP, resulting in significant tumor growth inhibition compared with a group injected with the same boron dose of L-BPA (Fig. 3).
 |
|
| Fig. 2. |
Boron concentrations in the cells at 2.5, 6, and 24 hours after incubating with PBC-IP and L-BPA.
|
 |
|
| Fig. 3. |
Tumor growth curves of U87MG xenograft mouse models after thermal neutron irradiation.
|
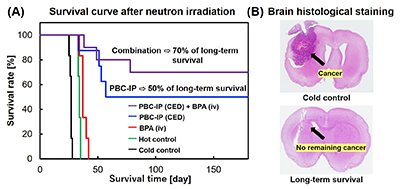
We conducted convection-enhanced delivery (CED) of PBC-IP to the localized brain tumor site of rat glioma models, followed by neutron irradiation. The median survival time of the group treated with L-BPA was 37 days, while 50% of the rat treated with the PBC-IP survived even after 180 days, demonstrating a remarkably extended survival effect (Fig. 4A). Moreover, 70% of the rat treated with a combination of PBC-IP and L-BPA survived after 180 days. Histological staining of the brains of long-term surviving rats showed there was no remaining tumor cells (Fig. 4B).
In summary, the BNCT using PBC-IP demonstrated a high tumor growth inhibition effect in a human glioblastoma xenograft mouse model. By using the CED method, the tumors were disappeared in 70% of the rat glioma model, while using only 1/50th of the typical boron agent dose. Glioblastoma is known as a refractory cancer the five-year survival rate of which is less than 10%. Conventional therapies, including surgery, chemotherapy, and radiotherapy, are low effective against glioblastoma, and an effective treatment has not been established yet. This study could provide a novel approach for the treatment of glioblastoma and have a significant impact on malignant cancer treatment using BNCT.
| [1] | F. Nakagawa, H. Kawashima, T. Morita, H. Nakamura, Cells 2020, 9, 1615. |
| [2] | K. Nishimura, S. Harrison, K. Kawai, T. Morita, K. Miura, S. Okada, H. Nakamura, Bioorg. Med. Chem. Lett. 2022, 72, 128869. |
| [3] | K. Nishimura, H. Kashiwagi, T. Morita, Y. Fukuo, S. Okada, K. Miura, Y. Matsumoto, Y. Sugawara, T. Enomoto, M. Suzuki, et al., J. Control. Release 2023, 360, 249-259. |
Tokyo Tech News : https://www.titech.ac.jp/english/news/2023/067174