Latest Research
- 2023.11.01
- Yoshizawa-Sawada Group
Development of a Polyaromatic Nanotool for Selective Dipeptide Recognition
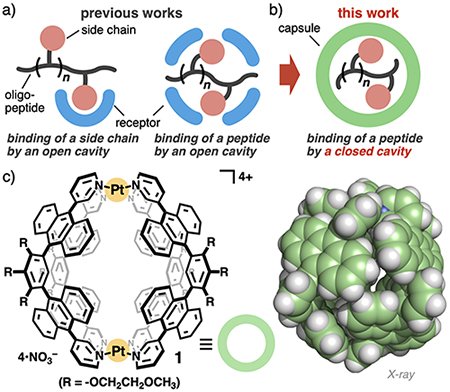
Peptides provide plenty of derivatives, depending on the number, kind, and order of amino acid residues, flexible frameworks, and high hydrophilicity. Owing to these properties, the precise recognition of oligopeptides by synthetic receptors remains extremely difficult, as compared with other biomolecules. The previously reported, synthetic receptors can be divided into two recognition types: the binding of a single or two side chain(s) or the partial/full inclusion of a peptide (Figure 1a).[1] In all cases, the receptors use open cavities for the biomolecular recognition. Therefore, the strict distinction of number and kind of the amino acid residues of oligopeptides as well as the selective binding of target oligopeptides from mixtures have been seldom demonstrated so far. Here we propose a new strategy for the selective recognition of oligopeptides using a closed cavity of a synthetic capsule (Figure 1b).
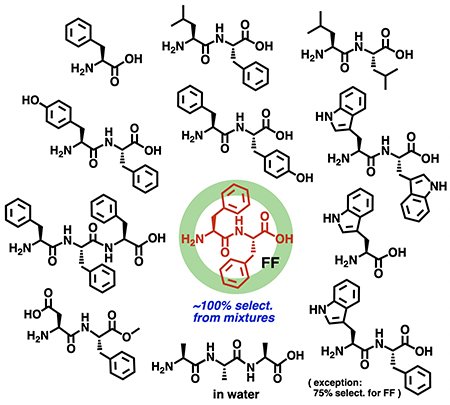
Aqueous polyaromatic capsule 1 employed in this study possesses a spherical cavity (∼1.3 nm in diameter and ∼580 Å3 in volume), surrounded by multiple polyaromatic panels (Figure 1c).[2-4] Unlike previous open receptors, the virtually closed cavity allows the capsule to recognize oligopeptides, based on the number and kind of amino acid residues, with excellent selectivity (∼100% selectivity), except for only two substrates (yet ≥75% selectivity). Actually, we revealed the selective and quantitative binding of one molecule of phenylalanine dipeptide (FF) by capsule 1, from complex mixtures with various amino acids and oligopeptides in water at room temperature (Figure 2). Detailed experimental (ITC, NMR, and IR) analyses and theoretical studies clarified that multiple host-guest CH-π and hydrogen-bonding interactions in the complementary cavity are key driving forces for the unusual high selectivity. Furthermore, selective emission detection of dipeptide FF could be accomplished using capsule 1 and a fluorescent coumarin dye in water.[5]
 |
|
| Figure 2. | Selective binding of phenylalanine dipeptide (FF) by polyaromatic capsule 1 from mixtures of various amino acids and oligopeptides in water. |
| [1] | L. Catti, R. Sumida, M. Yoshizawa, Coord. Chem. Rev. 2022, 460, 214460. |
| [2] | N. Kishi, Z. Li, K. Yoza, M. Akita, M. Yoshizawa, J. Am. Chem. Soc. 2011, 133, 11438-11441. |
| [3] | 1) M. Yamashina, T. Tsutsui, Y. Sei, M. Akita, M. Yoshizawa, Science Adv. 2019, 5, eaav3179; 2) K. Niki, T. Tsutsui, M. Yamashina, M. Akita, M. Yoshizawa, Angew. Chem. Int. Ed. 2020, 59, 10489-10492; 3) H. Dobashi, L. Catti, Y. Tanaka, M. Akita, M. Yoshizawa, Angew. Chem. Int. Ed. 2020, 59, 11881-11885; 4) R. Sumida, Y. Tanaka, K. Niki, Y. Sei, S. Toyota, M. Yoshizawa, Chem. Sci. 2021, 12, 9946-9951. |
| [4] | 1) M. Yamashina, M. Akita, T. Hasegawa, S. Hayashi, M. Yoshizawa, Science Adv. 2017, 3, e1701126; 2) S. Kusaba, M. Yamashina, M. Akita, T. Kikuchi, M. Yoshizawa, Angew. Chem. Int. Ed. 2018, 57, 3706-3710. |
| [5] | M. Shuto, R. Sumida, M. Yuasa, T. Sawada, M. Yoshizawa, A Closed Cavity Strategy for Selective Dipeptide Binding by a Polyaromatic Receptor in Water, JACS Au 2023, 3, 2905-2911 (https://pubs.acs.org/doi/10.1021/jacsau.3c00484). |