Latest Research
- 2024.03.01
- Yamamoto-Imaoka Group
Design and synthesis of 'alloy sub-nanoparticle' showing the unprecedented functions
When materials are finely chopped and reduced in size, their reactivity and physical properties undergo significant changes. Such dream-like phenomena are prominently observed when the size reaches to the sub-nano scale (around 1 nanometer), which is even smaller than the conventional nano scale. Generally, the atoms constituting a material are classified into those exposed on the surface and those hidden within the structure. The former can directly contribute to the adsorption and desorption of reactants, having high reactivity. Especially in ultrasmall particles of sub-nanoscale size, known as 'sub-nanoparticles (SNPs)', almost all of their constituent atoms are exposed on the particle surface, minimizing the presence of unused atoms that cannot contribute to reactions. In other words, miniaturization of a material to sub-nanoscale size can maximize the 'cost-performance' of its element. Furthermore, SNPs exhibit the remarkable potential, as they demonstrate their prowess in alloying which combines multiple types of metal elements. In the sub-nanoscale, even metal elements that would normally form phase-separation in bulk state are blended at the atomic level [1]. Therefore, in sub-nano science, it is expected to elucidate the properties of previously undiscovered alloy materials.
Then, synthesis of 'alloy SNPs' which multiple metal elements are mixed in the sub-nano scale have been technically difficult. For instance, when atoms are simply thrown into a liquid phase and stirred, gravitational forces between the atoms lead to their aggregation, resulting in particle growth. The particles formed in this process always follow a statistical distribution, making it almost impossible to precisely control the number of constituent atoms and the elemental composition as desired. Therefore, in order to overcome this obstacle, we have developed 'atom hybridization method (AHM)' using the nano-sized 'template' which realizes formation of particles within them [2]. In this method, we have adopted the 'dendrimer' as the template macromolecule. This dendrimer possesses imine units that have Lewis basicity within their structure, allowing them to accumulate Lewis acidic metal salts through complexation reactions. While conventional dendrimers also have this feature, our original dendrimers have the noteworthy characteristic not found in conventional ones. That is, there is a ranking of Lewis basicity among the imines contained within a dendrimer. Consequently, metal salts preferentially coordinate to the inner imines in the dendrimer, and the number of metal salts accumulated can be controlled depending on the equivalent amount of metal salt added. Furthermore, by chemically reducing these dendrimer complexes, the atoms accumulated within the dendrimers assemble, achieving the synthesis of SNPs with a predetermined atomicity. Additionally, when various metal salts are added, those with higher Lewis acidity are preferentially accumulated in the inner part of a dendrimer, while weaker metal salts are accumulated in the outer one. Thus, it is realized to synthesize of alloy SNPs with precisely controlled not only the number of constituent atoms but also the elemental composition.
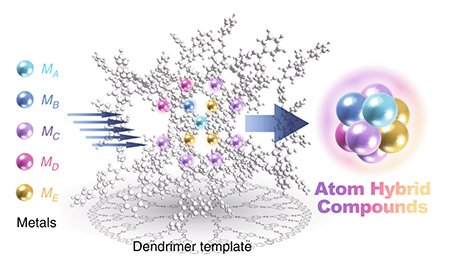 |
|
| Figure 1. Synthesis of alloy SNPs using the dendrimer ('AHM'). |
We have successfully synthesized alloy SNPs containing coinage metal elements such as gold (Au), silver (Ag), and copper (Cu) using AHM [3]. In this work, Au, Ag, and Cu salts were gradually accumulated from the inner part of the dendrimer in the order of increasing Lewis acidity (similar to the order of Olympic medals). By chemically reducing this dendrimer complex, we achieved the synthesis of Au-Ag-Cu alloy SNPs. We confirmed that the obtained particles have a controlled diameter of approximately 1 nanometer by scanning transmission electron microscopy (STEM) observation. Additionally, it was simultaneous demonstrated that these particles are composed of the three elements Au, Ag, and Cu using energy-dispersive X-ray spectroscopy (EDS) analysis. It is noteworthy that these three elements were blended in a SNP at the atomic level while they form phase-separation at bulk sizes. As these SNPs exhibit extremely high surface activity in general, they tend to aggregate immediately if left unattended. To prevent this, we immobilized the particles on support materials such as carbon and collect them as powder samples. This allows us to stabilize the SNPs for long time, making them suitable for various applications such as catalysis.
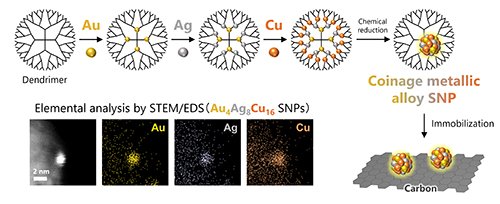 |
|
| Figure 2. Synthesis of Au-Ag-Cu alloy SNPs by 'AHM'. |
We applied the obtained Au-Ag-Cu alloy SNPs as catalysts for the oxidation of cyclohexene. In the case using conventional catalyst, this reaction has proceeded by strong oxidants or severe reaction conditions such as high temperature and pressure, resulting in hydroperoxide as an intermediate with ketones and alcohols as the main products. In contrast, in this work, we challenged the catalytic reaction under very mild conditions of 80 °C at atmospheric pressure using only oxygen molecules as the oxidant. Initially, we investigated the size effect of catalyst activity using bulk Cu, Cu nanoparticles (with the diameter of about 50 nanometers), and synthesized Cu SNPs as catalysts. As a result, miniaturization of the Cu catalyst to sub-nano size significantly improved its catalytic performance. Furthermore, a selective production of hydroperoxide which is a high-energy substance not typically obtained, was achieved. Hydroperoxide is an unstable substance that quickly decomposes under severe reaction conditions, but stable generation was made possible by the extremely mild catalytic reaction realized in this work. Moreover, alloying Au and Ag atoms with Cu SNPs further enhanced the catalytic activity, resulting that Au-Ag-Cu ternary alloy SNPs exhibited the highest catalytic performance. We investigated the factors contributing to the high activity of these alloy SNPs through both experimental and theoretical approaches. The results suggest that each element within the particles plays a specific role during the catalytic reaction, and the synergistic effects of these elements contribute to the high reaction activity. Specifically, Cu atoms primarily catalyze the reaction, Au atoms electronically activate the Cu atoms, and Ag atoms promote the desorption of reaction products. This synergistic activation by the three elements is a phenomenon observed for the first time in these alloy SNPs, which contrasts with bulk or nano particles that form phase-separation.
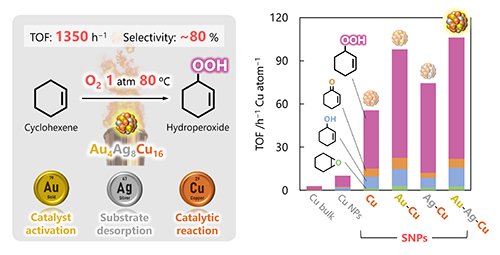 |
|
| Figure 3. | Catalytic performance of Au-Ag-Cu alloy SNPs over cyclohexene oxidation. |
As described above, we have succeeded in developing new alloy sub-nano catalysts with significantly higher activity than conventional catalysts, operating under much milder conditions. This achievement demonstrates that the potential to customize catalytic reactions at will by appropriately designing the elements constituting SNPs. Furthermore, such SNP catalysts, which use only a very small number of metal atoms and show the high function at low temperatures, are expected to be valuable materials from the perspectives of resource and energy issues. In the future, this may lead to the creation of next-generation environmental materials that consume minimal resources and energy.
References
| 1. | M. Inazu, Y. Akada, T. Imaoka, Y. Hayashi, C. Takashima, H. Nakai, K. Yamamoto, Nature Commun. 2022, 13, 2968. |
| 2. | T. Tsukamoto, T. Kambe, A. Nakao, T. Imaoka, K. Yamamoto, Nature Commun. 2018, 9, 3873. |
| 3. | T. Moriai, T. Tsukamoto, M. Tanabe, T. Kambe, K. Yamamoto, Angew. Chem. Int. Ed. 2020, 59, 23051. |



