Latest Research
- 2024.04.01
- Nakamura-Okada Group
Development of Ring-Expanding Skeleton Editing Technology for Heterocycles
Synthetic methods for novel heterocyclic compounds are crucial tasks in the development of pharmaceutical and agrochemical products. A methodology known as skeletal editing, which allows for the efficient synthesis of a diverse array of derivatives, has gained much attention in recent years.1 This involves molecular transformations that edit the skeleton of readily available heterocyclic compounds, including ring expansion or contraction, or the exchange of atoms (Fig. 1). As a result, transformations of ring structures at the level of individual atoms in the late-stage of synthetic process are becoming feasible.
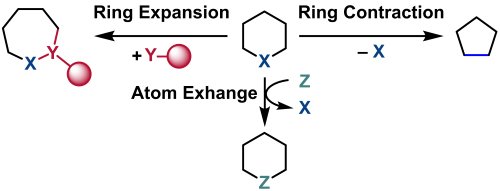 |
|
| Fig. 1. Concept of skeletal editing |
Heteroaromatic compounds known as 1,2-azoles, which bear relatively weak nitrogen-heteroatom bonds within their rings, have been used in ring expansion reactions that proceed through the cleavage of these bonds. Previous methods relied on highly reactive species such as rhodium carbenoids2 and free carbenes,3 which raised concerns about undesired side reactions. Additionally, extending the substrate scope remains a challenge. For example, the insertion of a simple methylene group has required multiple steps,4 necessitating the development of a more efficient method for this transformation.
In this study, we have demonstrated ring expansion of isoxazoles 1, a member of the 1,2-azoles, using a zinc carbenoid (EtZnCH2I) generated from diiodomethane and diethyl zinc (Fig. 2) 5,6). This methylene insertion into the N-O bond proceeded under mild conditions to afford a variety of 1,3-oxazines 2 from multi-substituted or fused isoxazoles. Density functional theory (DFT) calculations were performed to analyze the reaction mechanism, and we elucidated that this reaction proceeds through the following elementary processes: 1) N-alkylation, 2) cleavage of the N-O bond, and 3) cyclization.
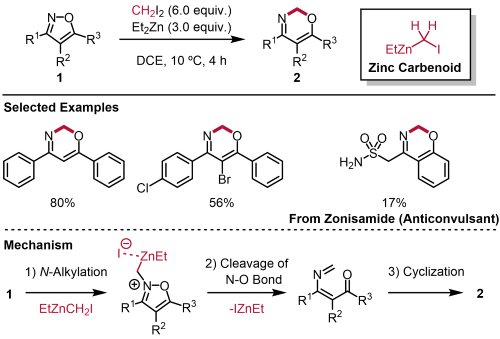 |
|
| Fig. 2. | Ring expanding skeleton editing of isoxazoles using zinc carbenoid |
The developed method was applicable not only to isoxazoles but also to various heterocyclic compounds 3 (Figure 3). Specifically, methylene insertion into the N-S bond in isothiazoles, as well as the N-N bond in pyrazoles and indazoles, was successfully demonstrated. Furthermore, this transformation also included five-membered oximes, which are not heteroaromatic compounds, to produce its ring-expanded products.
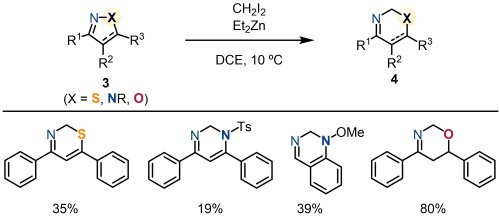 |
|
| Fig. 3. Application to other 1,2-azoles and a cyclic oxime |
In summary, we have developed a methylene insertion reaction into nitrogen-heteroatom bonds using zinc carbenoids, providing a wide variety of ring-expanded heterocycles. This reaction proceeds under mild conditions and provides the products in a single step, making it useful for the skeletal editing of 1,2-azoles and related heterocycles. We are currently exploring the expansion of the scope of heterocycles and zinc carbenoids.
References
| 1. | J. Jurczyk, J. Woo, S. F. Kim, B. D. Dherange, R. Sarpong, M. D. Levin, Nat. Synth. 2022, 1, 352-364. |
| 2. | (a) J. R. Manning, H. M. L. Davies, Tetrahedron 2008, 64, 6901-6908; (b) A. N. Koronatov, N. V. Rostovskii, A. F. Khlebnikov, M. S. Novikov, J. Org. Chem. 2018, 83, 9210-9219. |
| 3. | E. E. Hyland, P. Q. Kelly, A. M. McKillop, B. D. Dherange, M. D. Levin, J. Am. Chem. Soc. 2022, 144, 19258-19264. |
| 4. | C. Kashima, Y. Tsuda, S. Imada, T. Nishio, J. Chem. Soc. Perkin Trans I. 1980, 1866-1869. |
| 5. | M. Tsuda, T. Morita, H. Nakamura, Chem. Commun. 2022, 58, 1942-1945. |
| 6. | M. Tsuda, T. Morita, Y. Morita, J. Takaya, H. Nakamura Adv. Sci. early view (2023), DOI: 10.1002/advs.202307563. |



