Latest Research
- 2024.06.03
- Kitaguchi Group
Thermostable OpenGUS enzyme switch for homogeneous immunoassay development
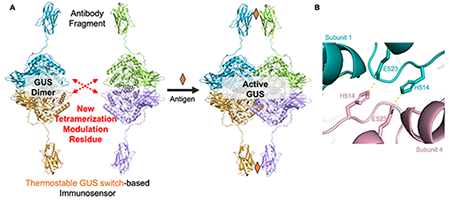
Immunoassays are widely used for analyte detection in diagnosis, food safety control, and environmental monitoring. The traditional immunoassays, such as ELISA, often require multiple steps for the separation or recovery of analytes from samples, complicating the testing process. Conversely, homogeneous immunoassays simplify this by allowing all reactions to occur within a single solution. Previously, our lab developed a β-glucuronidase (GUS) enzyme switch (OpenGUS) and antibody fusion-based biosensor for small molecule detection [1]. The reassembly of the OpenGUS was triggered by the antibody-antigen binding, which led to the activation of the GUS. However, the stability of the GUS switch was not ideal when the reaction temperature increased. This limitation restricted its application as an enzyme switch in immunoassays performed in environments without sufficient temperature controls. Therefore, a GUS switch variant with improved thermostability at physiological or high temperatures would broaden its practical applications. In this latest study [2], we developed a thermostable variant of the GUS switch, named GUSTR3-DLW, and more importantly, identified the critical role of H514 residue in controlling GUS tetramerization and the combination of the interface mutations in GUS switch development (Fig. 1).
This study began by using hyperthermostable GUSTR3 as a base for enzyme switch generation, incorporating mutations at the short interface that had been effective in other GUS variants. However, the mutations (M516K/Y517W or M516K/Y517E) at the short interface of GUSTR3 did not yield positive results in the homogeneous OpenGUS immunosensor framework for caffeine detection suggesting the insufficient separation of the GUSTR3 tetramer.
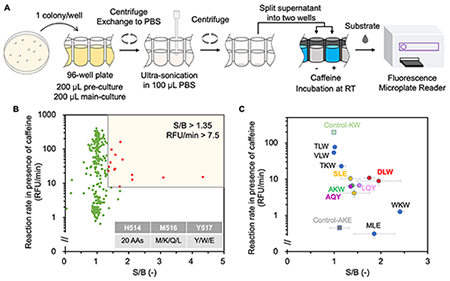
Therefore, we started exploring other critical residues not on the short interface that aid tetramerization to introduce additional mutations. We identified a polar interaction between H514 and E523 (Fig. 1B), which may form four salt bridges in a GUS tetramer. To find the most effective mutation combination, we performed a screening of a combinatorial library. A new high-throughput screening method for the selection of functional GUS enzyme switch mutants based on the caffeine-homogenous immunosensor design was established (Fig. 2A). A total of 283 valid clones (growth-confirmed) were subjected to the screening process (Fig. 2B). Clones that met the selection criteria were re-evaluated in triplicate and subsequently sequenced (Fig. 2C). The purified GUSTR3-DLW variant exhibited the best signal-to-background (S/B) ratio, aligning with the screening result.
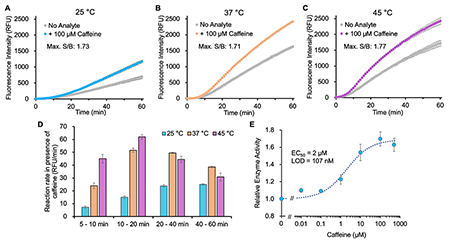
Thermostability is an important property for evaluating GUSTR3-based homogeneous immunosensors in this study. The previously developed OpenGUS [1] exhibited reduced enzyme activity at temperatures greater than 25 °C; however, the reaction rate of GUSTR3-DLW at 45 °C was four times faster than that at 25 °C (Fig. 3). This improved stability makes the GUSTR3-DLW a promising enzyme switch for the development of immunoassays for point-of-care testing. Recently, we began developing the next generation of the OpenGUS probe that can use label-free antibodies for antigen detection, which would be an alternative to conventional ELISA. The importance of the H514 mutation was also demonstrated in this OpenGUS probe design, significantly reducing background tetramerization [3]. Returning to this study, the GUSTR3-DLW switch exhibits a broader stable working temperature range compared to previously reported GUS switches, making it a versatile tool for developing thermostable homogeneous immunosensors and immunoassays for both in vitro and in-cellulo applications in the future.
| [1] | Su, J., Beh, C., Ohmuro-Matsuyama, Y., Kitaguchi, T., Hoon, S. and Ueda, H. (2019). Creation of stable and strictly regulated enzyme switch for signal-on immunodetection of various small antigens. J. Biosci. Bioeng., 128, 677-682. |
| [2] | Zhu, B., Qian, C., Tang, H., Kitaguchi, T. and Ueda, H. (2023) Creating a thermostable β-glucuronidase switch for homogeneous immunoassay by disruption of conserved salt bridges at diagonal interfaces. Biochemistry, 62, 309-317. |
| [3] | Zhu, B., Yamasaki, Y., Yasuda, T., Qian, C., Qiu, Z., Ueda, H. and Kitaguchi, T. (2023) Customizable OpenGUS immunoassay: a homogeneous detection system using split β-glucuronidase and label-free antibody. Jxiv, September 26, 2023. DOI: https://doi.org/10.51094/jxiv.511 |