Latest Research
- 2024.09.20
- Yoshizawa-Sawada Group
Terpene-Based Capsule: A Nanotool for Efficient Chirality Transfer
The majority of biological architectures (e.g., DNA and protein) are composed of chiral molecles, such as saccharides and amino acids (Figure 1a), to efficiently transfer and maintain essential chiral information in nature. Whereas synthetic chiral cavities are expected to demonstrate efficient chirality transfer in artificial systems, the elaborate molecular designs and multistep synthetic processes have been required for the cavities. In this study, we developed a new capsule providing a terpene-based chiral cavity, capable of transferring its chirality into various synthetic dyes upon encapsulation in water. Terpenes, chiral biosubstrates from plants (Figure 1b), are readily available molecules with relatively hydrophobic and rigid frameworks. However, the use for artificial chiral cavities remains unexplored.
 |
|
| Figure 1 |
(a) biorelated chiral molecules and (b) a terpene used in this study.
|
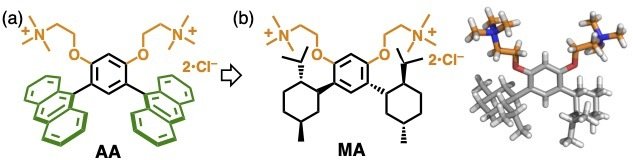
Based on bent anthracene-based amphiphile AA previously developed by our group (Figure 2a),[1,2] we designed new bent amphiphile MA, which incorporates two terpene units (i.e., p-menthane) (Figure 2b). The synthesis of chiral subunit MA and its enantiomer (MAE) was completed in six steps starting from (-) or (+)-menthol.
 |
|
| Figure 2 |
Bent amphiphiles (a) AA and (b) MA bearing terpene frameworks.
|
Micellar capsule (MA)n was spontaneously and quantitatively formed upon simple addition of MA to water at room temperature. (Figure 3a, left). DLS and molecular modeling studies revealed the formation of hexamer (MA)6 with a spherical terpene core (Figure 3b). Efficient chirality transfer from capsule (MA)n to organic dye TPE were demonstrated through the guest uptake, by manual grinding, water addition, and filtration (Figure 3a, right). The combination of DLS, UV-visible, NMR, and molecular modeling studies suggested the formation of (MA)21•(TPE)12 with an average core diameter of ~4 nm (Figure 3c). The UV-visible and CD analyses of (MA)n•(TPE)m revealed efficient optical chirality transfer from (MA)n to the dye aggregates (TPE)m (Figure 3d,e). Capsule (MA)n also bound planar polyaromatic as well as bulky fluorescent dyes (Figure 3f) in the cavity and subsequently displayed chirality transfer-based CD bands in water.
 |
|
| Figure 3 | (a) Formation of terpene capsule (MA)n in water and its encapsulation of TPE dye. (b) Optimized structures of capsule (MA)6 and (c) host-guest composite (MA)21•(TPE)12. (d) UV-visible spectrum (r.t., H2O) of (MA)n•(TPE)m and (e) CD spectra of (MA or MAE)n•(TPE)m. (f) Organic dyes studied herein. |
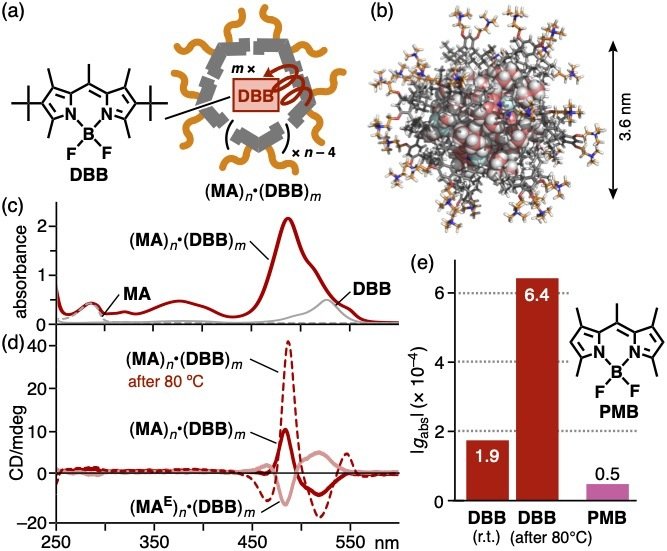
Amplification of chirality transfer efficiency was observed by applying a thermal stimulus to the host-guest composite. Host-guest composite (MA)n•(DBB)m were obtained from MA and BODIPY derivative DBB (Figures 4a,b). The UV-visible and CD spectra showed the Cotton effect in the 450-550 nm region, derived from the encapsulated (DBB)m (Figure 4c,d). When the aqueous solution of host-guest composite (MA)n•(DBB)m was heated at 80 ºC, the intensity of the Cotton effect significantly increased. On the basis of the absorption asymmetry factor (|gabs|), the guest-based CD band increased ~3-fold through the thermal stimulus (Figure 4e). This unusual behavior was not observed with the other host-guest composites.
 |
|
| Figure 4 | (a) Host-guest composite (MA)n•(DBB)m and (b) its optimized structure (n = 14 and m = 10). (c) UV-visible spectrum (r.t., H2O) of (MA)n•(DBB)m and (d) the CD spectra before/after heating. (e) |gabs| values of host-guest composites (MA)n including dyes DBB and PMB. |
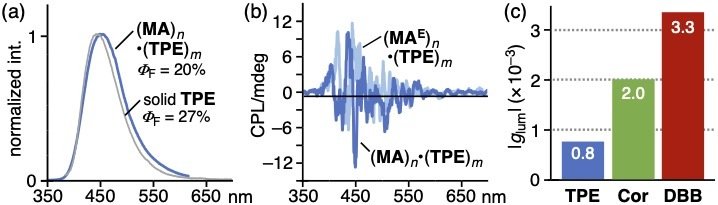
The PL and CPL properties of the host-guest composites were elucidated under ambient aqueous conditions. The aqueous solution of (MA)n•(TPE)m emitted relatively strong blue fluorescence (ΦF = 20%; Figure 5a). The CPL spectra of (MA)n•(TPE)m and its enantiomer displayed roughly symmetric mirror-like bands (Figure 5b). The absolute emission asymmetry factor (|glum|) was relatively high (0.8 × 10-3; Figure 5c). Other host-guest composites also exhibited high CPL activities. These results also indicated efficient optical chirality transfer from capsule (MA)n to the bound dyes.
 |
|
| Figure 5 |
(a) PL and (b) CPL spectra (r.t., H2O) of (MA)n•(TPE)m. (c) |gabs| values of host-guest composites (MA)n including dyes TPE, Cor, and DBB.
|
In summary, we have developed a novel chiral capsule utilizing terpene frameworks, which successfully encapsulates organic dyes in water.[3] The resulting host-guest composites exhibited distinct chiroptical properties derived from the bound achiral dyes. This strategy allows for the facile introduction of chirality to various achiral dyes without elaborate synthetic modification, highlighting its potential application to polymer materials and catalyst.
References
| [1] | K. Kondo, A. Suzuki, M. Akita, M. Yoshizawa, Angew. Chem. Int. Ed. 2013, 52, 2308-2312. |
| [2] | a) M. Yoshizawa, L. Catti, Acc. Chem. Res. 2019, 52, 2392-2404; b) M. Yoshizawa, L. Catti, Proc. Jpn. Acad. Ser. B 2023, 99, 29-38. |
| [3] | Y. Hashimoto, Y. Tanaka, D. Suzuki, Y. Imai, M. Yoshizawa, J. Am. Chem. Soc. 2024, 146, 23669-23673 (https://pubs.acs.org/doi/10.1021/jacs.4c07193). |



