Latest Research
- 2025.04.07
- Yamamoto-Imaoka Group
Atomic-Level Precision Synthesis of Metal Clusters Using Biomolecular Templates
In the field of nanoscience, techniques enabling the control of metal particles (clusters) at the sub-nanometer scale (less than 1 nm) have garnered considerable attention. At these dimensions, even smaller than typical metal nanoparticles (approximately 2-10 nm), the electronic structures and surface atom arrangements deviate significantly from those of bulk metals or larger nanoparticles, giving rise to unique catalytic activities and quantum effects [1]. However, methods for synthesizing and isolating such "sub-nanoclusters" have remained limited. While size-selected cluster generation under ultra-high vacuum conditions and the use of organic molecular templates such as dendrimers [2, 3] or cyclic multinuclear metal complexes [4] have been explored, these approaches face challenges such as large-scale equipment requirements and limited design flexibility. Consequently, a versatile and precise method for the broad synthesis of clusters has yet to be established.
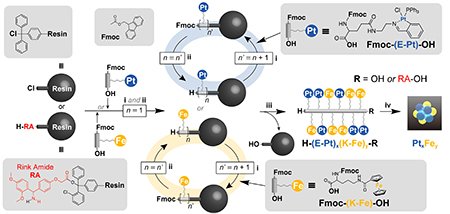
The core concept of our newly proposed approach [5] lies in the use of solid-phase peptide synthesis (SPPS) to design and synthesize metallopeptides--peptides bearing metal complexes on their side chains--and subsequently subjecting them to thermal decomposition to obtain metal clusters with high atomic precision (Fig. 1). SPPS is a widely used technique in biochemistry and pharmaceutical development that enables the automated, sequence-specific assembly of amino acids. The innovative aspect of this study is the deliberate repurposing of SPPS for the synthesis of ultra-small metal clusters. By sequentially linking amino acids functionalized with platinum (Pt) or iron (Fe) complexes, we can assemble peptides in a manner akin to molecular building blocks that precisely position metal atoms. Upon thermal decomposition of the peptide backbone, clusters with defined atom counts and compositions are formed on a carbon support.
 |
|
| Figure 1. | Overview of metal and alloy cluster synthesis using peptide-based templates. |
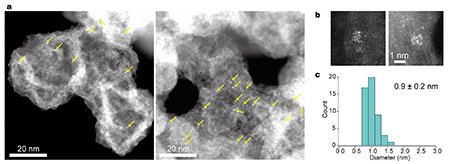
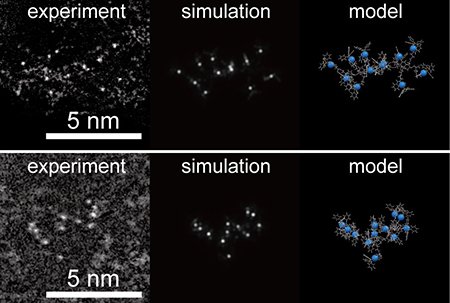
In SPPS, amino acids are linked to a solid-phase resin, with successive steps involving coupling, capping, and deprotection to suppress side reactions. We carefully selected ligands that are both stable under acidic and high-temperature conditions and easily removable upon final decomposition. Several amino acids bearing Fe and Pt complexes were synthesized and assembled in tailored sequences, such as a 12-residue Pt-complexed peptide (Fig. 2), or alloy precursors with alternating Fe and Pt complexes. The final thermal decomposition step, conducted under hydrogen or nitrogen atmospheres, removes the peptide framework and facilitates the aggregation of metal atoms on the support, yielding clusters approximately 1 nm in diameter (Fig. 3).
 |
|
| Figure 2. | High-resolution TEM image of a 12-residue Pt-complexed peptide and corresponding structural model. |
Sub-nanometer metal clusters are known to exhibit unprecedented catalytic activities. In the toluene oxidation reaction [6], Pt clusters synthesized via SPPS demonstrated higher yields than commercially available Pt catalysts, highlighting their efficiency even in trace amounts. We also succeeded in synthesizing Pt-Fe alloy clusters using the same strategy, which may find applications in oxygen reduction catalysts (e.g., fuel cell electrodes) or magnetic materials. A key advantage of SPPS lies in its capability to incorporate multiple metal species in arbitrary stoichiometries. Conventional alloy cluster synthesis often leads to particle coarsening or phase separation when targeting thermodynamically unstable compositions. In contrast, the use of peptide side chains as individual metal atom carriers enables the easy realization of rare or metastable compositions.
The greatest significance of this method lies in its ability to repurpose widely available SPPS as a platform for sub-nanocluster synthesis. Since the technique falls within the scope of standard automated synthesis equipment, it offers a promising route to accelerate the discovery of new functional materials through the precise control of cluster size and composition. Although current limitations include synthesis scale per peptide strand and the diversity of alloy types, integration with automated synthesizers and robotics could enable large-scale screening in the future. Moreover, further exploration from a biochemical perspective--such as the self-assembly or higher-order structuring of metallopeptides--could open new possibilities. For instance, imparting more complex spatial arrangements may enhance the selectivity of specific catalytic reactions.
The precise control of sub-nanoclusters promises to have a profound impact not only on catalytic chemistry but also in the development of novel functional materials for optics and magnetism. The "SPPS + metal complex" approach introduced in this study offers an unprecedented combination of flexibility and atomic-level precision, making it a highly promising direction for future cluster synthesis.
Reference
| [1] | T. Imaoka, T. Toyonaga, M. Morita, N. Haruta, K. Yamamoto, Chem. Commun. 2019, 55, 4753-4756. |
| [2] | K. Takada, M. Morita, T. Imaoka, J. Kakinuma, K. Albrecht, K. Yamamoto, Sci. Adv. 2021, 7, eabd9887. |
| [3] | M. Wakizaka, A. Atqa, W.-J. Chun, T. Imaoka, K. Yamamoto, Nanoscale 2020, 12, 15814-15822. |
| [4] | T. Kambe, A. Watanabe, M. Li, T. Tsukamoto, T. Imaoka, K. Yamamoto, Adv. Mater. 2020, 32, 1907167. |
| [5] | M. Inazu, Y. Akada, T. Imaoka, Y. Hayashi, C. Takashima, H. Nakai, K. Yamamoto, Nature Commun. 2022, 13, 2968. |
| [6] | Q. Zou, Y. Akada, A. Kuzume, M. Yoshida, T. Imaoka, K. Yamamoto, Angew. Chem. Int. Ed. 2022, 61, e202209675. |