Latest Research
- 2025.04.18
- Nakamura-Okada Group
Development of magnetic field-induced drug release system and magnetic caged calcium based on iron oxide nanoparticles and hydrogels
Functional materials have been developed for magnetic field-induced drug release in deep tissues. In magnetically responsive drug release systems, magnetic nanoparticles that generate localized heat under an alternating magnetic field (AMF) have been widely used[1]. The generated heat induces drug release from thermo-responsive drug carriers surrounding the magnetic nanoparticles. Moreover, magnetically responsive materials have potential as chemical tools to control signal transduction through receptor activation. We report herein a novel AMF-responsive material applicable to both drug release systems and chemical tools[2].
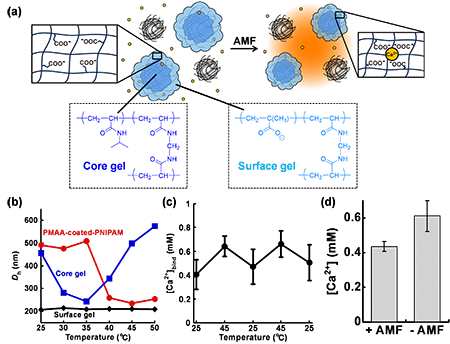
In this study, we developed the AMF-responsive drug release system consisting of polymer-coated iron oxide nanoparticles (PCIOs) and a drug carrier gel based on poly(N-isopropylacrylamide) (PNIPAM) (Figure 1a). The carrier gel can be easily replaced with a gel designed according to the intended use because the PCIO and the carrier gel are separate components.
The PCIOs were synthesized by polymerizing hydrophilic monomers on the surface of oleic acid-coated iron oxide nanoparticles. The presence of the polymer layer was confirmed by transmission electron microscopy and Fourier transform infrared (FT-IR) spectroscopy. The polymer coating constituted 1.5% of the total weight. As the drug carrier gel, P(NIPAM-co-AA), which is a copolymer of NIPAM and acrylic acid (AA), was synthesized by emulsion polymerization. P(NIPAM-co-AA) exhibited reversible volume transition between 37-45°C under physiological osmolarity, indicating it works near body temperature. The neutral small molecule PM-546 showed the highest loading efficiency into P(NIPAM-co-AA), and its release was promoted at 45°C. When the suspension containing PCIO and the PM-546-loaded gel was exposed to 12 cycles (3 hours) of AMF exposure (16 mT and 386 kHz), the one cycle of which was defined as 10 min exposure and 5 min rest, the solution temperature increased from 25°C to 39°C (Figure 1b). Quantification of PM-546 in the solution after 4 cycles (1 hour) and 12 cycles (3 hours) of AMF exposure showed significant release enhancement compared to that without AMF exposure (Figure 1c), demonstrating that the system functioned as an AMF-induced drug release platform.
Furthermore, we explored the potential of this platform as a chemical tool by replacing the drug carrier gel with a different gel. A representative chemical tool, caged calcium, has been used to control intracellular Ca2+ signaling by releasing Ca2+ upon exposure to UV-visible light[3]. However, it is challenging to apply this approach to deep tissues due to limited tissue penetration of light and to control extracellular Ca2+ which fluctuates in the sub-mM range. To address these issues, we designed a magnetic caged calcium system that could regulate Ca2+ concentrations under AMF exposure by combining PCIO with a thermo-responsive Ca2+-binding gel (Figure 2a). We hypothesized that volume transition of polycarboxylic acids induced changes in the distance between carboxy side chains, resulting in modulation of the Ca2+ binding affinity. We synthesized a PMAA-coated-PNIPAM gel by coating PNIPAM with poly(methacrylic acid) (PMAA) via precipitation polymerization.
FT-IR spectra and zeta potential measurements confirmed that PMAA-coated-PNIPAM possessed features of both PNIPAM and PMAA. PMAA-coated-PNIPAM exhibited temperature-dependent volume changes, with a size of approximately 500 nm at temperatures below 35°C and 235 nm at 45°C (Figure 2b). Furthermore, Ca2+ binding increased with rising temperature, showing reversible calcium binding between 25°C and 45°C (Figure 2c). Since neutralization titration curves of PMAA-coated-PNIPAM did not change with temperature, the binding strength for Ca2+ was regulated by changes in the distance between the carboxy side chains of the PMAA gel induced by the volume transition. When AMF was applied for 1 hour to the magnetic caged calcium composed of PCIO and PMAA-coated-PNIPAM, the Ca2+ concentration decreased by 0.18 ± 0.06 mM, indicating that Ca2+ concentration could be controlled by AMF exposure (Figure 2d).
In conclusion, we developed two systems using PCIOs and PNIPAM-based hydrogels to control drug release and calcium concentration by AMF exposure. The combination of PCIO and PM-546-loaded P(NIPAM-co-AA) accelerated PM-546 release upon AMF exposure, demonstrating potential for embedded devices of drug release targeting deep tissues. Moreover, by combining PCIO with a thermo-responsive Ca2+-binding gel (PMAA-coated-PNIPAM), we succeeded in reducing the Ca2+ concentration by 0.18 ± 0.06 mM under AMF exposure. Although further optimization is needed, this method is promising as a chemical tool for investigating biological functions involving extracellular Ca2+, such as immune responses and symbiosis with gut microbiota[4].
Reference
| [1] | Gavilán, H.; Avugadda, S. K.; Fernández-Cabada, T.; Soni, N.; Cassani, M.; Mai, B. T.; Chantrell, R.; Pellegrino, T. Chem. Soc. Rev. 2021, 50, 11614-11667. |
| [2] | Okada, S.; Takashima, Y.; Nakamura, H. ACS Appl. Polym. Mater. 2025, DOI: 10.1021/acsapm.5c00652 |
| [3] | Ellis-Davies, G. C. R. Acc. Chem. Res. 2020, 53, 1593-1604. |
| [4] | Owen, J. L.; Cheng, S. X.; Ge, Y.; Sahay, B. Mohamadzadeh, M. Semin. Cell Dev. Biol. 2016, 49, 44-51. |