Latest Research
- 2025.05.01
- Fukushima-Shoji Group
Triptycene-Based Supramolecular-Scaffold-Directed Assembly of Pentacene Chromophores to Promote Singlet Fission
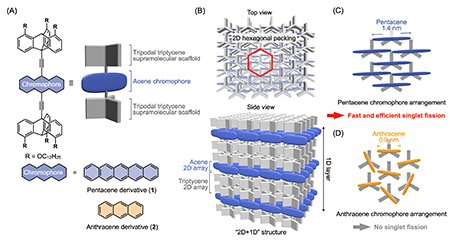
Two-dimensional (2D) assemblies of π-electron chromophores are expected to exhibit intriguing properties arising from their structural dimensionality. Their morphologies are highly compatible with various electronic devices, making them a subject of interest in diverse fields such as supramolecular chemistry, materials science, and organic electronics. However, the spontaneous assembly of molecules into 2D structures rarely occurs. We have proposed an approach using a supramolecular scaffold[1] to directly construct 2D assemblies using diverse molecules. Supramolecular scaffolds are molecular building blocks that can form characteristic assembled structures even after chemical modifications. Recently, we have focused on 2D supramolecular scaffolds based on tripodal triptycenes[2]. These tripodal triptycene supramolecular scaffolds can be used to assemble different molecular units or polymers in a 2D fashion[3]. The key to this approach is the fact that tripodal triptycene derivatives form highly ordered 2D sheet-like structures through packing in a nested hexagonal arrangement that fills the free volume around their skeletons, with these sheets aligning one-dimensionally to form layers, thereby forming a "2D+1D" structure.
In this study, we investigated the triptycene-based supramolecular scaffold directed 2D assembly of a pentacene chromophore capable of singlet fission. Singlet fission (SF) is a phenomenon in which a single excited singlet state (S1) gives rise to two free excited triplet states (T1 + T1) through the formation of an excited triplet pair [(T1T1)*]. Since SF can theoretically generate two excitons from a single photon, it has attracted significant attention to improve performance in thin-film solar cells and optoelectronic devices. To achieve efficient SF in the solid state, two conditions must be satisfied. Firstly, it should be ensured that sufficient orbital overlap occurs between chromophores to allow formation of an excited triplet pair. The second is to provide sufficient space around the chromophores to allow the conformational changes necessary for the excited triplet pair to dissociate into two excited triplet states. However, it is not straightforward to rationally design a structural arrangement that satisfies both of these conditions.
Considering the geometric features of the 2D assembly structure, when the pentacene chromophore is positioned at the bridgehead position of the triptycene, the distance between chromophores is estimated to be approximately 4 Å (Fig. 1). In this assembly structure, it is possible to design space that allows for orbital overlap between adjacent chromophores while permitting conformational changes. Thus, we designed and synthesized a sandwich-type derivative (1) by introducing a pentacene unit into the bridgehead position of a triptycene, and investigated its assembly structure and photophysical properties (Fig. 1). A sandwich-type molecular design was adopted to suppress strong stacking between chromophores. Additionally, we synthesized a smaller anthracene derivative (2) as a reference compound (Fig. 1A).
By casting chloroform solutions of these compounds onto substrates, we successfully prepared films with the expected 2D+1D assembly structure (Fig. 1B). In the film of 1, X-ray diffraction analysis suggests that the pentacene chromophores have both sufficient interchromophore overlap to allow singlet fission and the necessary spatial arrangement to allow the excited triplet pairs generated to dissociate into two free triplets (Fig. 1C). A similar assembly structure was observed in the film of 2, while no overlap between chromophores was observed due to the shorter molecular length of the anthracene units (Fig. 1D).
Femtosecond transient absorption spectroscopy measurements revealed that in the film of pentacene derivative 1, an excited triplet pair is formed from the excited singlet state via fast, efficient SF (ΦSF = 88±5 %, kSF = 5.9×1012 s-1), and furthermore, the excited triplet pair efficiently dissociates into two excited triplet states (ΦT = 130±8.8 %). On the other hand, in the film of anthracene derivative 2, no SF is observed due to the absence of electronic interactions between the chromophores. These results clearly demonstrate that this tripodal triptycene-based supramolecular scaffold directs the pentacene chromophores to assemble into a structure that allows sufficient electronic interactions between the chromophores while maintaining the necessary space to accommodate conformational changes.
In this study, we successfully developed an organic thin film that achieves both fast singlet fission and highly efficient free triplet generation by using a triptycene-based supramolecular scaffold to assemble pentacene into a configuration that promotes singlet fission[4]. One future challenge is to orient the 2D pentacene array perpendicular to the substrate surface to fit into conventional device configurations. However, configurations with electrodes arranged horizontally, such as comb electrodes, would be compatible with the orientation of the chromophores in the films obtained, potentially opening up avenues for device applications. In addition, the results of this study demonstrate the usefulness of this approach using tripod-shaped triptycene supramolecular frameworks for designing 2D assemblies that can show the photonic functions of chromophores.
Reference
| [1] | F. Ishiwari, Y. Shoji, T. Fukushima, Chem. Sci. 2018, 9, 2028-2041.. |
| [2] | N. Seiki, Y. Shoji, T. Kajitani, F. Ishiwari, A. Kosaka, T. Hikima, M. Takata, T. Someya, T. Fukushima, Science 2015, 348, 1122-1126. |
| [3] | F. Ishiwari, Y. Shoji, C. J. Martin, T. Fukushima, Polym. J. 2024, 56, 791-818. |
| [4] | M. Fukumitsu, T. Fukui, Y. Shoji, T. Kajitani, R. Khan, N. V. Tkachenko, H. Sakai, T. Hasobe, T. Fukushima, Sci. Adv. 2024, 10, eadn7763. |