Latest Research
- 2025.06.13
- Kitaguchi Group
Achieving low-adverse-effect photoimmunotherapy
Photodynamic therapy (PDT) is attracting attention as an effective cancer treatment in which photosensitizers generate reactive oxygen species (ROS), such as singlet oxygen, upon exposure to highly tissue-penetrant near-infrared (NIR) light, selectively killing cancer cells. Among these, photoimmunotherapy (PIT), which employs antibodies conjugated with photosensitizers, leverages the high target specificity of antibodies to deliver photosensitizers directly to cancer cells. This strategy is conceptually similar to antibody-drug conjugates (ADCs), where cytotoxic drugs are linked to antibodies for targeted therapy. Unlike ADCs, however, PIT activates the photosensitizer mainly at the site of light exposure, lowing damage to healthy tissue and thus offering the potential for reduced adverse effects. However, conventional PIT still has safety concerns: even unbound antibody conjugates or those bound to Fc receptors on normal cells can produce ROS upon light exposure, potentially causing unintended cytotoxicity. To address this limitation, we developed a novel antibody-photosensitizer conjugate called the Quenching Photoimmunoconjugate (Q-PIC). In Q-PIC, ROS generation is strictly controlled, which occurs only when the antibody binds to the target cancer cell and is exposed to NIR light.
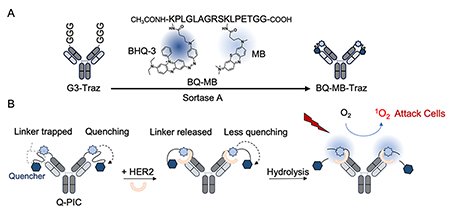
Q-PIC is prepared by ligating a peptide modified with methylene blue (MB) as the photosensitizer and Black Hole Quencher 3 (BHQ-3) as the quencher to the N-terminal region of the antibody heavy chain using transpeptidase Sortase A (Fig. 1A). In the absence of the target antigen, MB and BHQ-3 are connected via a peptide linker, and singlet oxygen generation is suppressed due to quenching from BHQ-3. Upon antigen binding, the "trapped" linker becomes "released" and undergoes hydrolysis, releasing BHQ-3 and thereby restoring MB's ability to generate singlet oxygen under NIR irradiation (Fig. 1B). By incorporating a protease-sensitive sequence into the linker, which is cleavable by proteases highly expressed in the tumor cells or microenvironment, Q-PIC is designed to selectively activate only after binding to the targeted cells and linker cleavage.
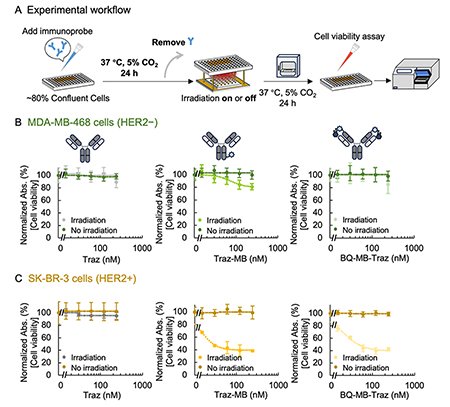
We performed in vitro experiment to evaluate the antigen-dependent cytotoxicity of Q-PIC before or after binding to the target antigen. Trastuzumab (Traz) is an antibody targeting human epidermal growth factor receptor 2 (HER2), which is overexpressed in certain breast cancers. We prepared Q-PIC from Traz (BQ-MB-Traz) and assessed its cytotoxicity against HER2-negative (MDA-MB-468) and HER2-positive (SK-BR-3) breast cancer cell lines.
Firstly, the unconjugated Traz, randomly MB-conjugated Traz (Traz-MB), and Q-PIC (BQ-MB-Traz) were added to the cells, followed immediately by light irradiation or no irradiation. This experiment mimics the situation in which unbound antibodies circulate in the bloodstream or before binding to the targeted cells in tissues (Fig. 2A). For both cell types, Traz and BQ-MB-Traz showed negligible changes in cell viability regardless of irradiation. In contrast, Traz-MB caused a dose-dependent decrease in viability under irradiation (Fig. 2B, C). These results suggest that BQ-MB-Traz has the expected minimal cytotoxicity without antigen binding, indicating a significantly improved safety profile compared to conventional probes.
Next, we incubated cells with Traz, Traz-MB, or BQ-MB-Traz for 24 hours before irradiation, followed with evaluation of cell viability (Fig. 3A). This experiment mimics the situation that the antibodies bind to the cells after sufficient probe exposures. Unbound antibodies were removed by washing prior to light exposure for seeing the effect from the bound antibody only. Under these conditions, unconjugated Traz did not affect viability regardless of HER2 status or irradiation. Traz-MB showed a dose-dependent decrease in viability in both cell types, with greater reduction in HER2-positive cells. Notably, cytotoxicity observed in HER2-negative cells likely reflects off-target effects mediated by Fc receptor interactions (Fig. 3B, C). In contrast, BQ-MB-Traz showed no cytotoxicity in HER2-negative cells regardless of irradiation, while in HER2-positive cells, it caused a dose-dependent viability reduction only upon irradiation. These results confirm that BQ-MB-Traz strictly induces cell death only in HER2-expressing cells.
In this study, we successfully developed a photosensitizer-conjugated antibody Q-PIC that enables antigen-dependent cancer cell killing. Future work will focus on optimizing the photosensitizer, quencher, and peptide linker design to further enhance therapeutic efficacy and safety, aiming toward practical applications in photoimmunotherapy.
| References: | |
| [1] | Zhirou Qiu, Bo Zhu, Takanobu Yasuda, Hiroshi Ueda, and Tetsuya Kitaguchi. N-terminal conjugation of a near-infrared photosensitizer to an antibody for cancer diagnosis and treatment. ACS Omega, 2025, in press. |