Latest Research
- 2025.07.04
- Shishido-Kubo Group
Development of a new method to significantly improve the efficiency of photopolymerization in polymer synthesis
Polymers are materials made out of long, repeating chains of molecules, and it is the interaction between these chains that dictate most of a polymer's physicochemical properties. In accordance with this intuitive understanding of polymers, which dates back to the 1930s, external forces acting on polymers are mainly regarded as destructive. For example, stretching a polymer can disentangle or break apart some of its constituent chains, weakening the overall material.
Over the past few decades, however, scientists demonstrated that external forces can have constructive effects on polymers. Mechanical forces and flow fields, if leveraged appropriately, have been shown to impart new functionalities in certain polymers by altering their phase, optical properties, and crystallinity. Despite substantial progress in this field--known as mechanochemistry--not much is known about how external forces can affect the growth behavior of polymers under external forces.
In this study, we investigated how flow fields induced by dynamic ultraviolet (UV) lighting can influence the process of photopolymerization, showcasing an interesting and versatile technique to control polymer synthesis.[1] Scanning UV light through a slit can boost the efficiency of photopolymerization reactions and produce polymers with higher molecular weight. This straightforward strategy induces molecular diffusion in the reactants that ultimately favor the formation of longer polymer chains while also saving massive amounts of energy, paving the way to more sustainable and efficient polymer synthesis.
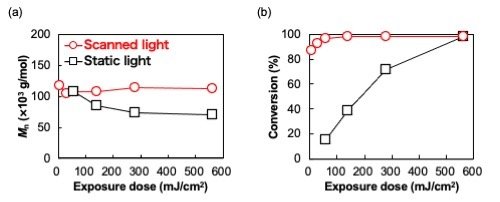
The photopolymerization reaction in question involved M6BACP as a monomer (the building block for the final polymer) and Irgacure 651, which is a photoinitiator. This last compound absorbs UV light and breaks down into reactive free radicals, which interact with the monomers and cause them to link together. But unlike typical photopolymerization processes, in which the entire monomer mixture is irradiated uniformly with UV light, we shone UV light through a slowly moving slit instead. Interestingly, this simple strategy had a profound effect on the resulting polymers, which we demonstrated through various comparative experiments. Photopolymerization with scanning UV light exhibited high molecular weight polymers, with a reduction of 90% of the required exposure dose compared to photopolymerization with static uniform light (Fig. 1).
 |
|
| Fig. 1. | Mn (a) and monomer conversion (b) of the M6BACP polymer photopolymerized by scanning light (red line) and static uniform light (black line) as a function of exposure dose.[1] |
The researchers theorize that UV light induces molecular flows with two notable effects. On the one hand, it causes growing polymers to diffuse slightly towards the yet-unirradiated area, as their concentration there is lower. This allows them to keep growing as radicals become available. On the other hand, radicals and monomers also diffuse, with the former being supplied to the unirradiated area and the latter to the irradiated area. As a result of this mutual diffusion, the radical-to-monomer concentration tends to become lower in irradiated areas, which minimizes the probability of termination reactions from taking place and dramatically enhances the polymerization efficiency, yielding long-chain polymer.
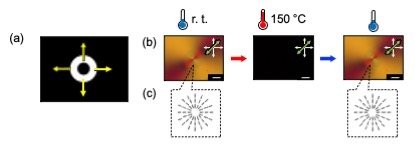
Furthermore, it has recently been discovered that the alignment of polymer main chains can be controlled in a flow field induced by moving light.[2] Photopolymerization using patterned light with an expanding circular pattern produced films with radially aligned molecules. When the film was heated to isotropic temperatures, the molecular alignment became random. However, it reoriented when cooled to room temperature (Fig. 2). These results demonstrate that polymer main chain alignment can be precisely and multidimensionally controlled using moving light.
 |
|
| Fig. 2. | 2D patterning of molecular alignment in polymer main chain and LC molecules. (a) Irradiation pattern used to induce 2D alignment patterning. (b) POM images with tint plate of the polymer coatings photopolymerized with 2D scanning light. (c) Schematic illustrations of the LC molecules in initial state and after the heating and cooling process of the polymer coatings.[2] |
Overall, this study not only provides important insights into photopolymerization reactions, but also showcases a convenient way of improving existing industrial processes and polymeric materials. The developed method significantly improves polymerization efficiency with a simple procedure of adding movement to the irradiation light, without changing existing compounds or reaction systems. This can reduce the energy cost of photopolymerization, which is used in various industrial applications, and is expected to be applied to manufacturing processes and foundational technologies for polymer synthesis. Worth noting, the observed advantages were found not only for M6BACP, but also for various commodity polymers, such as acrylates.
With any luck, these findings will pave the way to more sustainable ways to manufacture even better polymers.
| References: | |
| [1] | T. Ishiyama, Y. Kobayashi, H. Nakamura, M. Aizawa, K. Hisano, S. Kubo, A. Shishido, Macromolecules 57, 7430-7438 (2024). |
| [2] | H. Nakamura, T. Ishiyama, M. Sato, K. Tomida, M. Aizawa, K. Hisano, W. Nakano, S. Kubo, A. Shishido, Langmuir 41, 10552-10561 (2025). |



