Latest Research
- 2025.08.07
- Yamaguchi-Kuroki Group
Revealing dominant factors influencing electrocatalytic performance of metal sulfides for water electrolysis reaction
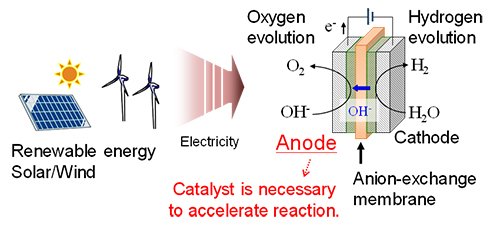
The global energy crisis and the imperative to mitigate climate change have positioned the development of green hydrogen as a strategic priority. Hydrogen, characterized by its high energy density and water as its sole combustion product, represents a compelling alternative to conventional fossil fuels. Among various production methods, water electrolysis--wherein water molecules are dissociated using electricity--offers a sustainable pathway, particularly when powered by renewable energy sources. Particularly, anion-exchange membrane (AEM) water electrolysis (Fig. 1) is performed in an alkaline environment at low temperatures (<100 °C) and considered as promising hydrogen production technology because its alkaline environment can employ inexpensive nonprecious metals, making it a cost-effective process for large-scale applications.[1] Additionally, AEM between the electrodes limits gas crossover and allows for high-pressure operation. Despite its promise, AEM water electrolysis faces a critical challenge: the oxygen evolution reaction (OER) at the anode side of water electrolysis shows insufficient reaction rates by using inexpensive nonprecious metal-based catalysts and thus remains a major kinetic bottleneck. Enhancing the efficiency of this half-reaction is essential for the widespread adoption of the AEM water electrolysis as a green technology for hydrogen production.
 |
|
| Fig. 1. | Overview of hydrogen production by AEM water electrolysis using renewable energies. |
In pursuit of more effective OER catalysts, transition metal sulfides have garnered significant attention due to their favorable electrochemical properties, including enhanced covalency and superior electrical conductivity relative to traditional metal oxides. However, the rational design of metal sulfide catalysts has been hindered by a lack of fundamental descriptors that can reliably predict catalytic performance, rendering the development process largely empirical.[2-5]
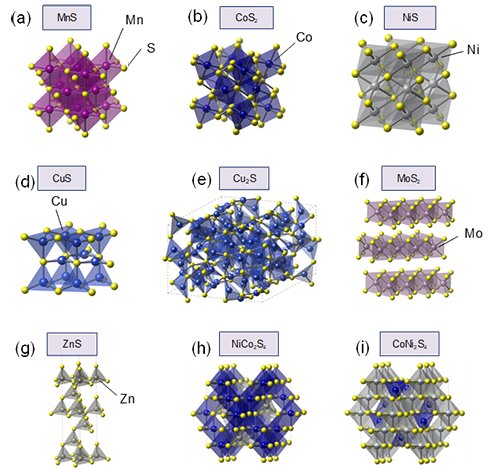
Addressing this gap, our research team has reported a pivotal advancement in the understanding of metal sulfide electrocatalysts for OER to identify a comprehensive descriptor for the OER activity of metal sulfides by systematically analyzing the key factors influencing their catalytic behavior. Herein, this study systematically investigated nine metal sulfides to elucidate the underlying principles governing their OER activity. We selected and synthesized the following metal sulfides incorporating various transition metals (Fig. 2), namely, MnS, CoS2, NiS, CuS, Cu2S, MoS2, ZnS, NiCo2S4, and CoNi2S4, and evaluated their electrocatalytic performance through a combination of experimental electrochemical techniques and density functional theory (DFT) calculations.
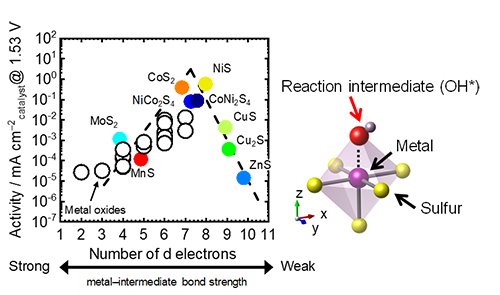
The analysis revealed a principal finding; i.e., a volcano-type relationship was found between OER activity and the number of d electrons in the metal atoms, with optimal performance occurring at 7-8 d electrons, as shown in Fig. 3. This trend reflects a balance in the adsorption strength of reaction intermediates: metals with fewer d electrons bind intermediates too strongly, while those with more d electrons bind them too weakly. This is the first report to establish that the number of d electrons serves as a predictive descriptor for the OER activity of metal sulfides, and this insight provides a robust framework for the rational design of high-performance catalysts. Collectively, these findings offer a transformative approach to catalyst development. By employing the number of d electrons as a design parameter, researchers can now predict the electrocatalytic potential of metal sulfide candidates with greater accuracy, thereby streamlining the discovery process and reducing associated costs.
As global investments in hydrogen infrastructure accelerate, this fundamental breakthrough lays the groundwork for the development of efficient and scalable catalyst materials, advancing the realization of a sustainable hydrogen economy. It is anticipated that water electrolysis will become a cornerstone technology for clean hydrogen production in the near future.[7]
The part of this paper is based on results obtained from a project commissioned by the New Energy and Industrial Technology Development Organization (NEDO). This study was carried out using the TSUBAME4.0 supercomputer at Institute of Science Tokyo.
| References | |
| [1] | Y. Sugawara, S. Sankar, S. Miyanishi, R. Illathvalappil, P. K. Gangadharan, H. Kuroki, G. M. Anilkumar and T. Yamaguchi, J. Chem. Eng. Jpn. 2023, 56, 2210195. |
| [2] | Y. Sugawara, K. Kamata, E. Hayashi, M. Itoh, Y. Hamasaki, T. Yamaguchi, ChemElectroChem 2021, 8, 4466-4471. |
| [3] | Y. Sugawara, S. Ueno, K. Kamata, T. Yamaguchi, ChemElectroChem 2022, 9, e202101679. |
| [4] | Y. Sugawara, K. Magofuku, T. Yamaguchi, Chem. Commun., 2025, 61, 8675-8678. |
| [5] | Y. Sugawara, Y. Nakase, G. M. Anilkumar, K. Kamata and T. Yamaguchi, Nanoscale Adv. 2025, 7, 456-466. |
| [6] | J. O. Bockris and T. Otagawa, J. Electrochem. Soc., 1984, 131, 290-302. |
| [7] | Y. Sugawara, T. Uchiyama, M. Shishkina, T. Yamaguchi, Catal. Sci. Technol. 2025, in press. |