Latest Research
- 2025.09.01
- Yoshizawa-Sawada Group
Opening Up Solution-State Chemistry of Unsubstituted Aromatic Polymers via Aromatic Micelles
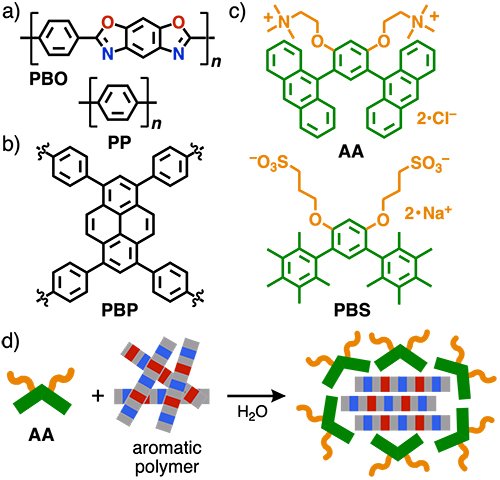
Polymeric materials are essential for our everyday lives. In the search for new functional materials, scientists have placed focus on polymers composed of rigid aromatic frameworks, displaying unique electronical/optical properties, heat resistance and mechanical advantages. However, due to their high rigidity and strong self-aggregation, aromatic polymers are insoluble in organic solvents and water, which hampers their analysis, processing and further development. Current solubilization methods mainly rely on laborious and costly installation of side-chains onto the polymer backbone, furthermore resulting in undesired property changes.
To address this issue, we studied the water-solubilization of such hard-to-handle, insoluble unsubstituted aromatic polymers utilizing bent aromatic amphiphiles (i.e., surfactants), developed in our laboratory (Figure 1a,b).[1] These amphiphiles consists of a hydrophobic bent aromatic framework featuring two hydrophilic side-chains. In water, the amphiphiles spontaneously and quantitatively assemble via the hydrophobic effect and π-stacking interactions into so-called aromatic micelles.[2] While their adaptable aromatic cavities have allowed the water-solubilization of various small-to-medium-sized guests (e.g., nanocarbons, dyes, metal complexes), their host functions towards giant polymeric guests had been completely unexplored so far. In this study, we could now demonstrate that anthracene-based bent amphiphile AA[1a] and its derivative PBS[1b] are capable of solubilizing various linear and spherical aromatic polymers without any substituents in water with high efficiency through encapsulation, opening up their solution-state analysis, processing, and host-guest applications (Figure 1c,d).
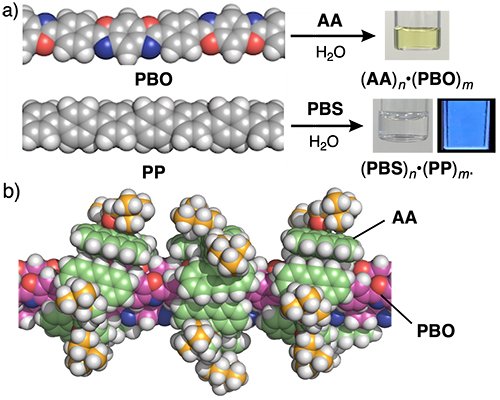
As a first target, commercially available linear aromatic polymer PBO (ZylonTM) was selected. Treatment of amphiphile AA and PBO using a grinding-sonication protocol yielded a clear yellow solution including (AA)n•(PBO)m (Figure 2a, top). The encapsulation of PBO by aromatic micelle AA was confirmed by the UV-visible analysis of the solution. Importantly, under the same conditions, the water solubilization efficiency was more than 50 times higher than that with a conventional alkane-based amphiphile. The host-guest structure was revealed via AFM analysis, displaying long, highly debundled wire-like objects. Based on this data, molecular modeling studies indicated that water-soluble (AA)n•(PBO)m is in average composed of a trimeric PBO bundle encircled by a tubular (AA)n shell, through effective hydrophobic effect and multiple CH-π/π-π interactions (Figure 2b). In a similar way, aromatic polymer PP was efficiently water-solubilized by using amphiphile PBS (Figure 2a, bottom). Interestingly, aqueous (PBS)n•(PP)m displayed strong blue emission (ΦF = 30%), owing to the efficient debundling of PP upon encapsulation.
 |
|
| Figure 2. | a) Water-solubilization of PBO and PP through encircling by amphiphiles AA and PBS, respectively. b) Optimized structure of (AA)n•(PBO)m. |
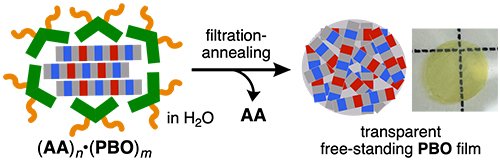
The encapsulated polymers could be easily released from the aromatic micelles and thus a free-standing PBO thin-film was prepared from the aqueous (AA)n•(PBO)m solution using a simple filtration-annealing protocol. FE-SEM-based surface analysis of the obtained thin-film showed an intertwined network of polymer fibers of 10-20 nm thickness, further confirming uptake of the polymer as thin bundles. The thickness of the obtained film was estimated to be ~600 nm.[3]
 |
|
| Figure 3. | Preparation of a free-standing PBO thin-film via a filtration-annealing protocol. |
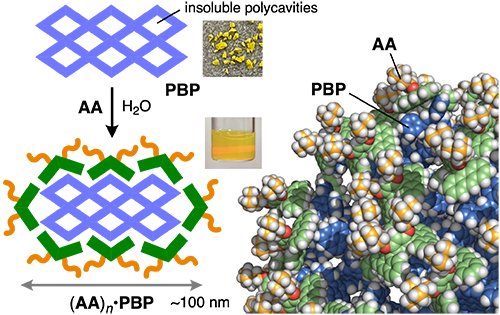
Based on these results, we next established the encapsulation of porous spherical aromatic polymer PBP, featuring a network of semi-flexible polycavities (< 2 nm; Figure 1b). The solubilization was again achieved using a simple grinding-sonication protocol (Figure 4). The resultant solution exhibited a prominent Tyndall effect, suggesting the dissolution of large polymer particles. Detailed structure analysis of the obtained host-guest composites in water revealed an average particle diameter of ~100 nm. Based on this data, molecular modelling suggested that a single PBP particle is encapsulated by (AA)n, interacting via multiple π-π and CH-π interactions on the PBP surface.
 |
|
| Figure 4. | Encapsulation of porous spherical aromatic polymer PBP by amphiphile AA and molecular modeling of a partial surface segment of (AA)n•PBP. |
Water-solubilized PBP furthermore acted as an aqueous polycavity host toward dyes and hydrocarbons. Dye DCM was loaded into the polycavities of PBP using a simple three-component grinding protocol with amphiphile PBS, yielding ternary composite (PBS)n•PBP•(DCM)m (Figure 5). The incorporation of DCM into the polycavities of PBP was confirmed by the observation of new absorption and fluorescence bands derived from DCM. Moreover, unprecedented four-component composite (PBS)n•PBP•(DCM)m•(cDec)p was obtained by stirring an aqueous solution of (PBS)n•PBP•(DCM)m with cyclodecane (cDec) (Figure 5, right). Co-incorporation of DCM and cDec resulted in an 8-fold increase in DCM-based fluorescence. In a similar way, quaternary host-guest composites were also obtained using an insoluble thiophene oligomer.[4]
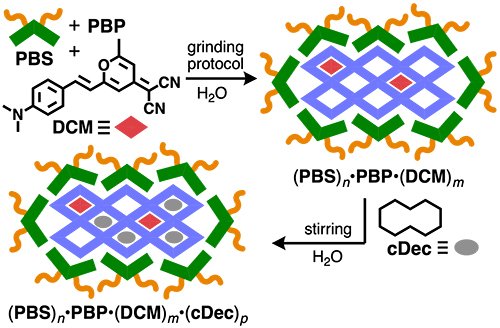 |
|
| Figure 5. | Formation of ternary and quaternary host-guest composites via encapsulation of dyes and dyes with hydrocarbons, respectively, into the polycavities of (PBS)n•PBP. |
In summary, we have developed an efficient method for the water-solubilization of various linear and spherical aromatic polymers without any substituents. The method is highly general and can be applied to a wide-range of polymeric structures. Through the water-solubilization of the insoluble polymers, we have achieved their in-solution analysis, processing, and host-guest applications. The uptake of other aromatic polymers as well as metal-organic and inorganic solids with well-defined designable polycavities by aromatic micelles and the development of their solution-state host abilities are our next research targets.
| References | |
| [1] | a) K. Kondo, A. Suzuki, M. Akita, M. Yoshizawa, Angew. Chem. Int. Ed. 2013, 52, 2308-2312; b) Y. Okazawa, K. Kondo, M. Akita, M. Yoshizawa, Chem. Sci. 2015, 6, 5059-5062. |
| [2] | a) M. Yoshizawa, L. Catti, Acc. Chem. Res. 2019, 52, 2392-2404; b) M. Yoshizawa, L. Catti, Proc. Jpn. Acad. Ser. B 2023, 99, 29-38. |
| [3] | S. Aoyama, L. Catti, M. Yoshizawa, Angew. Chem. Int. Ed. 2023, 62, e202306399. |
| [4] | S. Aoyama, L. Catti, M. Yoshizawa, Chem 2025, 11, 102616. |