Latest Research
- 2025.10.01
- Kamiya-Asanuma Group
Development of Fluorescence and Raman Probes Based on a Novel Scaffold
Recently, a wide variety of chemical probes have been developed and applied not only to life science research but also to clinical diagnostics and fluorescence-guided surgery. We have recently succeeded in developing fluorescence and Raman probes based on dyes with unique properties. Here, we present two of our recent studies.
The first study concerns the development of fluorescence and Raman probes based on a Coumarin-Hemicyanine (CHC) hybrid dye scaffold.
CHC is known as a ratiometric fluorescence probe, which enables quantitative fluorescence imaging by utilizing the ratio of short-wavelength fluorescence derived from the coumarin moiety and long-wavelength fluorescence from the entire CHC dye. Our group has previously developed CHC probes whose ratiometric fluorescence can be controlled by introducing an intramolecular nucleophile into the CHC dye, thereby triggering intramolecular cyclization [1]. Conventional CHC dyes exhibit fluorescence around 650 nm (deep-red); however, near-infrared (NIR) fluorescence is more advantageous for deep-tissue imaging owing to its improved tissue penetration [2]. Moreover, in Raman microscopy, which has recently attracted considerable attention, such dyes with red-shifted absorption properties can provide strong Raman signal detection when excited with NIR light sources [3].
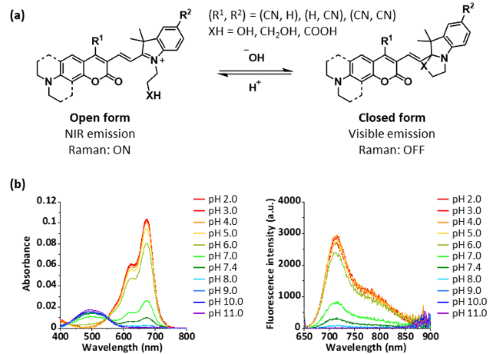
In this study, we developed a red-shifted CHC dye, 4CN-CHC, by introducing a cyano group into the CHC scaffold [4]. Since cyano groups are known to induce red shifts in various dyes when introduced at appropriate positions, we first synthesized a series of CHC derivatives with cyano substitution at different positions of the coumarin moiety (Fig. 1a). We found that substitution at the 4-position of the coumarin unit afforded the most pronounced bathochromic shift. Furthermore, similar to conventional CHC dyes, the newly developed 4CN-CHC dyes exhibited changes in their absorption and fluorescence properties through intramolecular cyclization, depending on the pH of the solution (Fig. 1b).
 |
|
| Fig. 1 | | (a) Chemical structure and scheme of intramolecular cyclization equilibrium of nitrile-substituted CHC dyes. (b) pH-dependent changes in the absorption and fluorescence properties of 4CN-JCHC-1. |
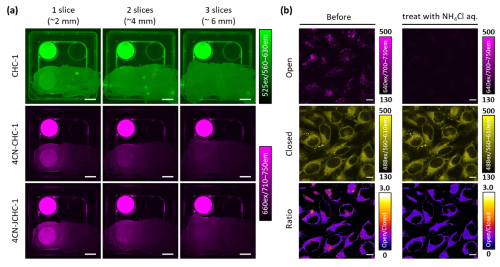
Next, we examined the tissue penetration of the >700 nm NIR fluorescence of 4CN-CHC. Fluorescence penetration was evaluated by placing layered slices of chicken breast meat as a tissue phantom over dye solutions. Whereas conventional CHC dyes showed a marked decrease in fluorescence intensity beyond two layers, 4CN-CHC fluorescence was still clearly detectable through three layers, demonstrating its superiority for deep-tissue imaging (Fig. 2a). In addition, we focused on one of the developed dyes, 4CN-JCHC-1, whose ratiometric fluorescence value exhibited an inflection point in the weakly acidic region, and investigated its potential for cellular imaging to monitor pH changes in lysosomes. Fluorescence images were acquired before and after the addition of NH4Cl solution, which is known to neutralize lysosomal pH. Upon NH4Cl treatment, a pronounced decrease in the long-wavelength fluorescence from the ring-opened form was observed, and corresponding changes in the ratiometric values were clearly visualized in ratio images comparing the short- and long-wavelength fluorescence (Fig. 2b).
 |
|
| Fig. 2 | | (a) Comparison of fluorescence tissue penetration between conventional CHC dyes and 4CN-CHC dyes. (b) Fluorescence imaging of lysosomal pH changes using 4CN-JCHC-1. |
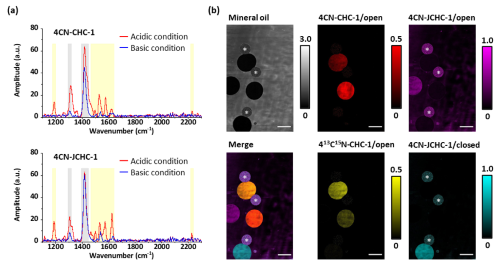
Furthermore, the red-shifted absorption properties and the nitrile triple-bond structure of 4CN-CHC make it suitable for Raman imaging, which detects molecular vibrations [3]. In particular, our group has previously demonstrated that Raman signal switching (off/on) can be controlled by changes in molecular absorption wavelengths [5,6]. We therefore hypothesized that the wavelength shifts induced by intramolecular cyclization of 4CN-CHC could also be exploited to regulate Raman signals. When we measured the coherent anti-Stokes Raman scattering (CARS) spectra of the ring-opened and ring-closed forms of 4CN-CHC, we observed a marked decrease in signal intensity for the closed form compared with the open form (Fig. 3a). This result indicates that Raman signal intensities of nitrile and C=C vibrations can be modulated through pH-dependent intramolecular cyclization.
Moreover, by taking advantage of the narrower peak width and richer spectral information of Raman spectra compared with fluorescence spectra, we demonstrated that multiple 4CN-CHC dyes, which are difficult to distinguish by fluorescence detection alone, could be simultaneously detected and imaged using CARS spectra (Fig. 3b).
 |
|
| Fig. 3 | | (a) CARS spectral changes induced by intramolecular cyclization of 4CN-CHC dyes. (b) Simultaneous imaging of multiple 4CN-CHC dye-encapsulated droplets by CARS microscopy. |
In summary, the probes developed here represent activatable probes with unique properties in both fluorescence and Raman imaging and are expected to serve as promising tools for bioimaging applications.
This work was carried out in collaboration with Professor Hideaki Kano at Keio University, Professor Kotaro Hiramatsu at Kyushu University, and Professor Yasuteru Urano at the University of Tokyo. Part of this research was performed using the TSUBAME 4.0 supercomputer at Institute of Science Tokyo.
As the second study, we introduce the development of novel enzyme-activatable-Raman probes based on BzBMN, which exhibits aggregation properties in aqueous solution.
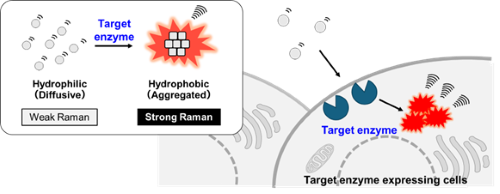
Fluorescent probes for detecting diverse enzyme activities in living systems have been widely utilized across a broad range of fields, from basic life science research to clinical studies [7]. However, due to the broad overlap of absorption and emission spectra, the number of enzyme activities that can be simultaneously visualized by fluorescence probes is limited to 4 to 5. In contrast, Raman imaging has attracted attention as a technique capable of simultaneously detecting a greater variety of molecular species, owing to its much narrower spectral linewidths compared with fluorescence [3, 5-6]. Inspired by our preliminary observation that dye aggregates, with their relatively high local concentrations, exhibit stronger Raman signals than their dispersed dyes, we designed and developed a new type of Raman probe for enzyme activity based on aggregation control (Fig. 4) [8].
 |
|
| Fig. 4 | | Schematic of the Raman probes for enzyme activity based on aggregation control. |
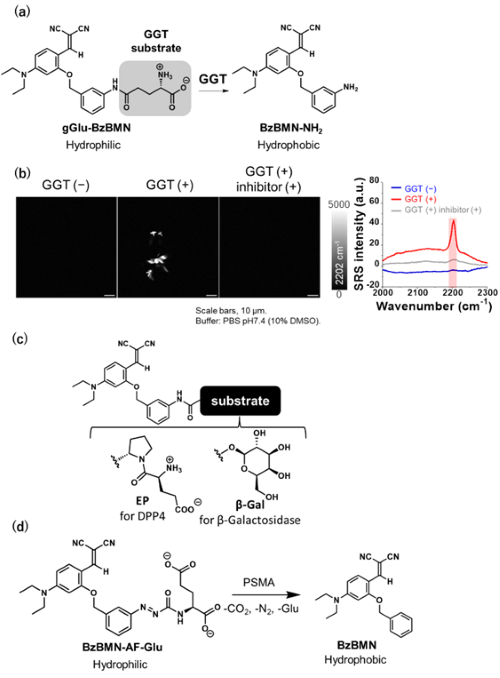
We focused on and selected BzBMN as a scaffold that forms aggregates in aqueous solution [9] by considering that its derivatives can be synthetically accessible, that BzBMN comprises two nitrile groups that give a characteristic Raman signals in a silent region with minimal cellular background, and that BzBMN exhibits aggregation-induced emission enhancement (AIEE) properties, making it easy to evaluate aggregate formation.
To introduce enzyme substrate moiety, we first synthesized BzBMN-NH2 with an amino group at benzene moiety of BzBMN and confirmed that it formed aggregation in aqueous solution. We then designed and synthesized gGlu-BzBMN by conjugating γ-glutamyl group to BzBMN-NH2, thereby developing a probe targeting γ-glutamyltranspeptidase (GGT), which is known to be overexpressed in specific cancer cells (Fig. 5a). Compared with BzBMN-NH2, gGlu-BzBMN showed improved water solubility, and Raman imaging of the respective solutions revealed that gGlu-BzBMN formed few aggregates. However, upon addition of GGT, BzBMN-NH2 was produced and formed aggregates that exhibited strong Raman signals in silent region (Fig. 5b). This reaction was suppressed by the addition of a GGT inhibitor, confirming that gGlu-BzBMN worked as a Raman probe capable of detecting GGT activity.
Similarly, we developed EP-BzBMN targeting dipeptidylpeptidase IV (DPP4) and βGal-BzBMN, targeting β-galactosidase activity. We also developed BzBMN-AF-Glu, which introduces glutamate via an azoformyl linker, enabling detection of prostate-specific membrane antigen (PSMA), a glutamate carboxypeptidase known to be overexpressed in prostate cancer cells (Fig. 5c, 5d). These results demonstrated that this probe design is applicable to various hydrolases.
 |
|
| Fig. 5 | | (a) Molecular structure of gGlu-BzBMN and its reaction scheme with GGT. (b) Raman imaging and spectra of gGlu-BzBMN reacted with GGT observed by stimulated Raman scattering (SRS) microscopy. (c) Molecular structures of Raman probes targeting DPP4 and β-galactosidase. (d) Molecular structure of a Raman probe targeting prostate specific membrane antigen (PSMA) and its reaction scheme with PSMA. |
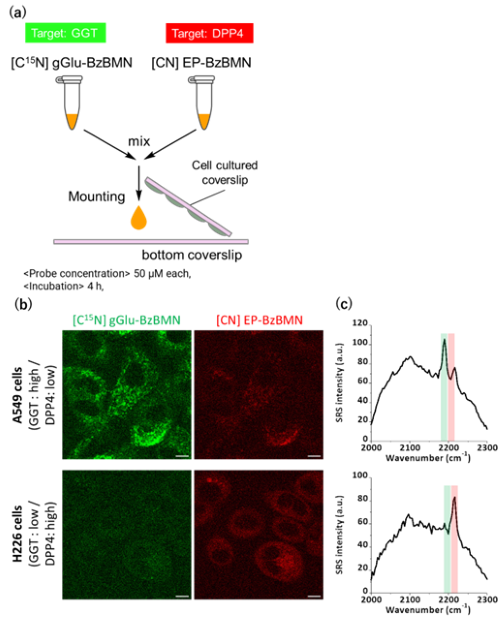
We then applied gGlu-BzBMN to A549 lung cancer cells, which highly express GGT, and observed bright puncta with strong Raman signals inside the cells. These puncta were scarcely observed in the presence of a GGT inhibitor or in H226 lung cancer cells, which express low levels of GGT, confirming that gGlu-BzBMN is also capable of detecting GGT activity in living cells. Likewise, EP-BzBMN and βGal-BzBMN successfully detected their respective target enzyme activities in live-cell imaging experiments.
Finally, we tried multiplexed imaging of two enzyme activities. We developed isotopically substituted derivatives of BzBMN and measured their Raman spectra, finding that the Raman shift varied according to the isotope used, allowing them to be spectrally distinguished. Using this strategy, we combined [C15N] gGlu-BzBMN (a GGT probe) with EP-BzBMN (a DPP4 probe) and applied the mixture to A549 or H226 cells. Raman imaging revealed that A549 cells displayed strong peaks derived from the C≡15N vibration (GGT probe), while H226 cells showed strong peaks derived from the C≡N vibration (DPP4 probe), enabling clear visualization of distinct enzyme activity patterns (Fig. 6).
 |
|
| Fig. 6 | | (a) Simultaneous Raman imaging of GGT and DPP4 activities using [C15N] gGlu-BzBMN and EP-BzBMN. (b) Raman imaging of GGT activity (green) and DPP4 activity (Red). (c) Corresponding Raman spectra. |
Compared with conventional Raman probes, developed probes in this study exhibit higher stability and greater flexibility in molecular design, making them promising tools for the simultaneous monitoring of diverse enzyme activities in life science research.
This work was carried out in collaboration with Professor Yasuyuki Ozeki and Professor Yasuteru Urano at the University of Tokyo.
| References | |
| [1] | H. Fujioka, S. Uno, M. Kamiya, R. Kojima, K. Johnsson and Y. Urano, Chem. Coomun. 2020, 56 (42), 5617-5620. |
| [2] | H. Li, Y. Kim, H. Jung, J. Hyun and I. Shin, Chem. Soc. Rev. 2022, 51 (21), 8957-9008. |
| [3] | L. Wei, Z. Chen, L. Shi, R. Long, A. Anazalone, L. Zhang, F. Hu, R. Yuste, V. Cornish and W. Min, Nature, 2017, 544 (7651), 465-470. |
| [4] | H. Fujioka, R. Sakamoto, K. Hiramatsu, Y. Murakami, M. Masaki, M. Kawatani, S. Matsumoto, R. Kojima, Y. Urano, H. Kano and M. Kamiya, Anal. Chem. 2025, 97 (32), 17589-17597. |
| [5] | H. Fujioka, J. Shou, R. Kojima, Y. Urano, Y. Ozeki and M. Kmiya, J. Am. Chem. Soc. 2020, 142 (49), 20701-20707. |
| [6] | H. Fujioka, M. Kawatani, S. Spratt, A. Komazawa, Y. Misawa, J. Shou, T. Mizuguchi, H. Kosakamoto, R. Kojima, Y. Urano, F. Obata, Y. Ozeki and M. Kamiya J. Am. Chem. Soc. 2023, 145 (16), 8871-8881. |
| [7] | K. Fujita, Y. Urano, Chem Rev. 2024, 124 (7), 4021-4078. |
| [8] | M. Okinaka, M. Kawatani, H. Fujioka, S. J. Spratt, H. Ito, Y. Misawa, R. Otake, A. Ishikawa, R. Kojima, Y. Urano, Y. Ozeki and M. Kamiya, Anal. Chem. 2025, 97 (35), 19057-19065. |
| [9] | Q. Dai, W. Liu, L. Zeng, C. Lee, J. Wu, P. Wang, CrystEngComm. 2011, 13, 4617. |



