Latest Research
- 2025.11.01
- Nishiyama-Miura Group
Development of smart nanomachines to overcome the challenges of adeno-associated viral vectors (AAV)
The key to successful gene therapy is the method to deliver the therapeutic genes into target cells. Viral vectors, which exploit the mechanism of viral infection, are widely used. Adeno-associated viral vectors (AAVs) are capable of transducing genes into various cell types and achieving long-term gene expression. AAVs have been clinically applied to treat intractable diseases such as spinal muscular atrophy, Duchenne muscular dystrophy, and hemophilia. However, the majority of adults naturally possess AAV-neutralizing antibodies (NAbs), and also AAV administration produces NAbs, limiting the patients eligible for treatment as well as the multiple administrations. Furthermore, high-dose AAV administration is toxic to the liver and kidney, resulting in fatalities in clinical trials. To address these challenges, approaches such as modifying the viral vector capsid with poly(ethylene glycol) (PEG) and incorporating AAV into delivery vehicles such as liposomes have been attempted. However, these approaches might result in reduced cellular uptake and gene transfer efficiency of AAV. Therefore, there is a strong demand for an AAV delivery system that can evade AAV from NAbs in the blood, suppress their accumulation in the liver, and control gene transfer into target cells.
In this study, we developed an AAV-loaded nanomachine composed of tannic acid (TA), a kind of polyphenols abundant in wine and tea, and synthetic polymers (Figure 1). TA interacts with biomolecules, such as proteins and AAV, through hydrophobic interactions and hydrogen bonds to form nanocomplexes. By incorporating PEG-poly(amino acid) block copolymer bearing a phenylboronic acid group in the side chain into the nanocomplex formed from TA and AAV, we constructed a core-shell AAV-loaded nanomachine with a PEG-covered surface. The phenylboronic acid forms a boronate ester with the galloyl group of TA. While stable at pH 7.4 in blood, the boronate ester dissociates in the acidic environment (~pH 5.5) of intracellular endosomes/lysosomesand, and the polymer is released, activating AAV. This is expected to avoid the reduced activity of AAV that can be caused by PEG modification.
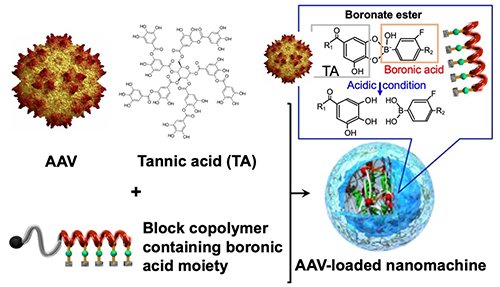 |
|
| Figure 1. | Schematic diagram of AAV-loaded nanomachines formed from TA and block copolymers bearing boronic acid moiety |
The nanomachines carrying AAV serotype 9 (AAV9) were 59 nm in size. Their in vitro gene expression of a reporter gene (luciferase, Luc) in HEK293 cells was examined, revealing that the nanomachines increased Luc expression per copy of AAV9, due to the inhibition of AAV9 proteasomal degradation. Furthermore, Luc expression in the presence of an anti-AAV9 antibody (neutralizing antibody) was significantly reduced in AAV9 alone and in the AAV9/TA complex, whereas the AAV9-loaded nanomachines maintained high Luc expression regardless of anti-AAV9 antibody (Figure 2). This is likely due to the polymer shell of the nanomachines protecting the loaded AAV from inactivation by the neutralizing antibody.
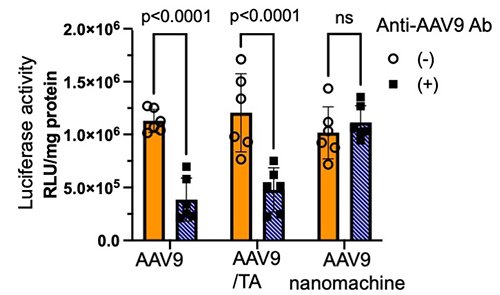 |
|
| Figure 2. | Effect of anti-AAV9 antibody on Luc expression activity of AAV9 in HEK293 cells |
Next, we evaluated the effect of neutralizing antibodies (NAbs) on in vivo gene transfer of AAV9 by administering serum containing NAbs to mice followed by AAV9 administration 10 minutes later. As expected, AAV9 alone and the AAV9/TA complex showed significantly reduced Luc expression activity after serum administration, whereas the AAV9-loaded nanomachines maintained high Luc expression activity (Figure 3). Furthermore, gene transfer efficiency in the brain and liver decreased to 5-15% in the AAV9 alone and AAV9/TA complex groups, whereas the AAV9-loaded nanomachine group maintained approximately 50-60% gene expression activity. This demonstrated that the AAV9-loaded nanomachines can prevent inactivation by NAbs in vivo (Figure 3). Furthermore, with regard to safety, liver toxicity markers (GOT and GPT) were significantly increased in the AAV9 alone and AAV9/TA complex groups, but these markers remained unchanged in the AAV9-loaded nanomachine group, demonstrating their reduced hepatic toxicity (Figure 4).
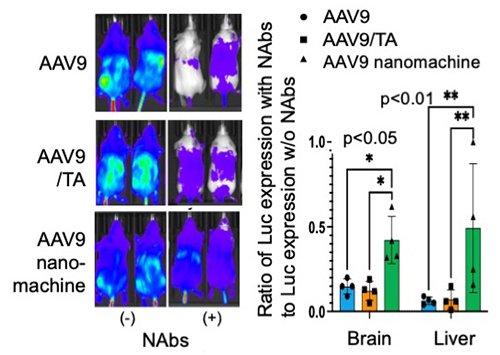 |
|
| Figure 3. | AAV9 gene expression in mice administered serum containing neutralizing antibodies (NAbs) |
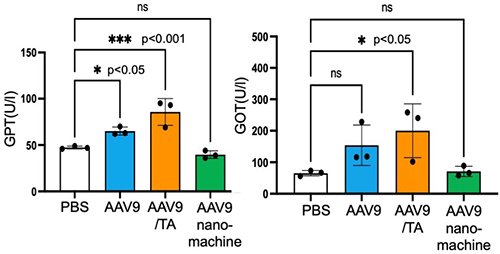 |
|
| Figure 4. | Evaluation of liver toxicity markers (GOT and GPT) after AAV9 administration |
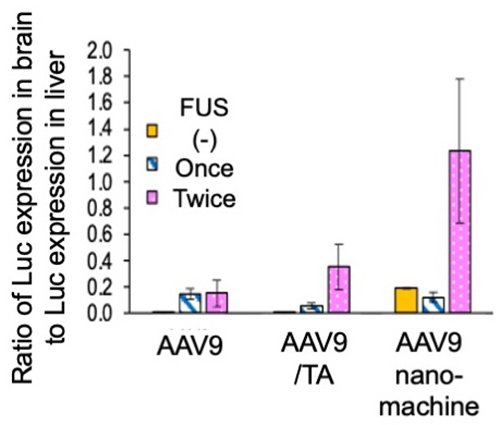
Finally, we focused on AAV9 gene transfer to the brain and evaluated the effect of focused ultrasound (FUS) irradiation using microbubbles to enhance the gene transfer efficiency in the brain. This method is expected to improve AAV9 expression efficiency in the brain by transiently increasing vascular endothelial permeability. The first ultrasound irradiation was performed 1 hour after AAV9 sample administration, and the second ultrasound irradiation was performed 23 hours later. The gene expression efficiency of AAV9 was evaluated as the ratio of gene expression efficiency in the brain to that in the liver. While ultrasound irradiation improved gene expression in all AAV9 samples, the AAV9-loaded nanomachines with twice FUS irradiations exhibited a six-fold increase in gene transfer efficiency in the brain (Figure 5).
 |
|
| Figure 5. | Selective gene expression of AAV9 in the brain by ultrasound irradiation (vs. Luc expression efficiency in the liver) |
As described above, we successfully avoided the clinical challenges of AAVs, such as inactivation by NAbs and side effects in normal tissues such as the liver. This technology is expected to improve the efficacy and safety of gene therapy and contribute to expanding the target diseases and increasing the number of patients. Furthermore, improved selectivity of gene expression in the brain may also bring its application to neurodegenerative diseases such as Alzheimer's disease. In recent years, advances in gene therapy, such as genome editing therapy using CRISPR, have led to growing expectations for improved functionality of viral vectors. We expect that this technology will contribute to cutting-edge medical care and intend to further improvement in its functionality.
Reference
Y. Honda, S. Nagao, H. Kinoh, X. Liu, N. Matsudaira, A. Dirisala, S. Nitta-Matsutomo, T. Nomoto, H. Hayashita-Kinoh, Y. Miura, T. Okada, N. Nishiyama, Adeno-associated virus self-assembled with tannic acid and phenylboronic acid-polymers to evade neutralizing antibodies and reduces adverse events. ACS Nano 19(8)7690-7706 (2025) (doi: 10.1021/acsnano.4c11085)



