Latest Research
- 2026.01.01
- Yamamoto-Imaoka Group
How Subnano Particles Selectively Engineer High-Performance Nanomaterials
Recent advances in nanotechnology have been remarkable, leading to the development of innovative functional materials such as smartphones, quantum devices, environmental energy-harvesting materials, and next-generation computers. In the pursuit of further performance enhancement and energy efficiency in such material development, one challenge remains constant: improvement without compromise. As a promising path toward this goal, attention is now turning to an emerging field--the world smaller than nanoscale: 'the subnano' regime. Subnano particles are ultrafine clusters composed of only a few to several tens of atoms, with sizes below just 1 nanometer. At first glance, they may appear to be nothing more than 'very small nanoparticles'. However, the world of subnano materials defies the established principles that hold true in the conventional nano size range. For example, when the size of matter is reduced to the subnanometer regime, the ordered crystalline structure is lost, giving rise to unusual geometric configurations, electronic states, and adsorption-desorption behavior that are not observed even in nanoparticles [1]. As a consequence, subnano particles exhibit physical and chemical properties that fundamentally challenge conventional element-based material science [2,3]. Moreover, the precise design and fabrication of advanced functional nanomaterials greatly benefit from the utilization of even smaller building units--namely, subnano particles. Here, we introduce an example of our research demonstrating the synthesis of carbon nanotubes (CNTs) using subnano particles as seeds [4].
Among various carbon nanomaterials with diverse properties, CNTs have attracted significant attention as a pioneering high-performance material. CNTs are extremely lightweight (approximately half the density of aluminum), while demonstrating mechanical strength surpassing steel and electrical and thermal conductivity exceeding copper. Because these properties strongly depend on diameter, number of walls, and structural parameters, developing a reliable, low-cost, and reproducible synthesis method to produce CNTs with targeted specifications remains a major challenge. Several synthesis routes have been reported, including catalytic chemical vapor deposition (CCVD), arc discharge, and laser ablation. Among them, we focused on the mechanism of CCVD. In conventional CCVD, metal catalyst nanoparticles are immobilized on a support material, where they decompose carbon-source gas molecules (hydrocarbons, alcohols, CO, etc.) and subsequently grow CNTs. Our approach, however, takes a distinctly different route. The key feature of our method is that metal subnano particles are dispersed as 'seeds' on the support, and during the reaction they undergo: ① aggregation upon heating to form metal nanoparticles, and ② subsequent catalytic growth of CNTs. These two steps proceed sequentially within the same reaction system, without external intervention. In other words, instead of preparing catalyst nanoparticles beforehand, the catalyst with the desired size forms spontaneously during the reaction, and CNTs naturally grow from it (Figure 1).
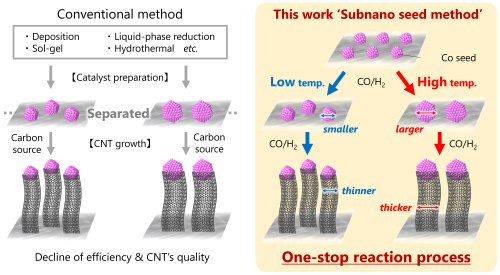 |
||
| Figure 1. | The growth scheme of CNTs using subnano particles as seeds. | |
For the synthesis of the subnano particles used as seeds, we employed a dendrimer-templated method utilizing a dendritic polymer. This dendrimer contains a total of 60 imine units that exhibit Lewis basicity, enabling coordination of up to 60 Lewis acidic metal ions through complexation reactions. Upon chemical reduction of this dendrimer-metal complex, the metal ions confined within the dendrimer framework are assembled and form a subnano particle, with a precisely controlled diameter of around 1 nm. The subnano particles obtained in this way are prone to rapid aggregation when left in solution; therefore, they are typically immobilized on support materials such as oxides and carbon. In this work, cobalt (Co) was selected as the metal species and was supported on amorphous silica. The synthesized Co subnano particles were characterized using scanning transmission electron microscopy (STEM) with atomic resolution and energy-dispersive X-ray spectroscopy (EDS). These analyses confirmed the presence of approximately 1 nm Co subnano particles dispersed on the silica support (Figure 2).
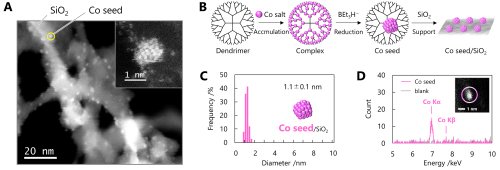 |
||
| Figure 2. | (A) STEM images, (B) preparation scheme, (C) the histogram of a diameter, and (D) EDS spectra of Co subnano particles (seeds). | |
Next, carbon nanotube (CNT) growth was carried out via CO/H₂ flow catalytic chemical vapor deposition (CCVD) using the synthesized Co sub-nano particles as seeds. Before the reaction, the Co seeds were immobilized on the silica support, and their surfaces were oxidized under ambient conditions. When exposed to a stream of high-temperature hydrogen, the particle surfaces were chemically reduced, while the interfacial bonds between the Co seeds and the silica support were simultaneously cleaved, enabling the seeds to become mobile on the silica surface. This mobility allowed the seeds to diffuse and aggregate, forming nanosized Co particles. In the absence of CO, aggregation would continue indefinitely; however, under CO-containing conditions, the Co nanoparticles begin functioning as catalysts for CNT growth once they reach a certain critical size. During this process, carbon atoms generated from the decomposition of CO at the Co nanoparticle surface diffuse beneath the particle, eventually causing the particle to lift from the silica support and continuously grow a CNT underneath (Figure 3). Consequently, the diameter of the resulting CNT is strongly dependent on the size of the catalytic metal particle. This mechanism is known as the tip-growth mode, and because further particle aggregation becomes negligible during growth, CNTs with highly uniform diameters can be obtained. These results clearly indicate that controlling the particle size of the catalytic Co nanoparticles--essentially, the degree of aggregation of the Co seeds--is crucial for precision CNT synthesis. If the interaction between the Co seeds and the support is too weak, excessive aggregation occurs; conversely, if the interaction is too strong, Co nanoparticles fail to form and CNT growth does not proceed. In this work, this delicate balance was achieved by identifying and applying the optimal support material. As a result, simple adjustments of reaction temperature and duration under ambient pressure conditions enabled precise control over CNT diameter. In other words, it is the precision of the subnano Co seeds that ultimately governs both the size of the resulting Co nanoparticles and the diameter of the CNTs they produce.
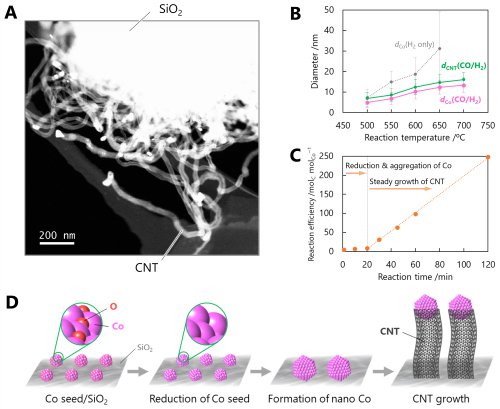 |
||
| Figure 3. | (A) A STEM image of CNTs grown by CCVD technique (600ºC, 1 h) using Co seeds. (B) The diameter of Co nanoparticles and CNTs formed by CCVD for 1 h at each reaction temperature. (C) The reaction efficiency of CNT growth by CCVD for 1 h. (D) The growth mechanism of CNTs by Co seeds. | |
Then, one fundamental question arises: must the seed necessarily be subnano-sized? Could a single metal atom serve the same purpose? The answer, based on our findings, is no--subnano particles are optimal for this CNT growth process. In the case of single Co atoms, the interaction with the silica support is considerably stronger, requiring significantly higher temperatures for hydrogen reduction to form catalytically active nanoparticles. As a result, while CNT growth occurred at temperatures as low as 500ºC when subnano seeds were used, no CNT formation was observed even at 600ºC when single atoms were used. At even higher temperatures, CNTs eventually formed, but with a broad diameter distribution, indicating loss of structural precision (Figure 4A).
Although the subnano seed method described above enables synthesis of CNTs with diameters exceeding 5 nm, the catalytic behavior can be further tuned through modification of growth parameters such as gas composition, catalyst support, and reaction temperature. Under optimized conditions, it becomes possible to suppress aggregation entirely and allow the subnano particles themselves to function directly as catalysts. Indeed, we successfully demonstrated the growth of ultrathin CNTs with diameters of approximately 1 nm (Figure 4B).
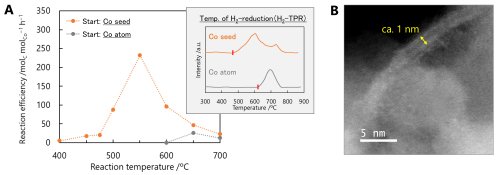 |
||
| Figure 4. | (A) The reaction efficiency of CNT growth by CCVD for 1 h using Co subnano particles or Co single atoms as seeds. (B) A STEM image of a CNT grown from a Co subnano particle. | |
These results demonstrate that by precisely designing subnanometer-sized seed particles and optimizing the support materials on which they are supported, it becomes possible to freely engineer a wide variety of nanomaterials. Many nanomaterials exhibit dramatic changes in their properties depending on their size and structure. Therefore, if synthesis can be achieved through such an extremely simple, one-stop, self-contained method as described above, it will significantly improve the efficiency of the manufacturing process. High-performance nanomaterials originating from particles composed of only a few atoms--although their starting point is remarkably small, they ultimately connect to the development of future technologies such as next-generation electronics, artificial muscles, AI devices, and quantum information systems. The era in which the ultrafine scale of just one nanometer--the subnano world--unfolds into groundbreaking innovations on a vast scale may not be far away.
| [1] | K. Yamamoto, T. Imaoka, Acc. Chem. Res. 2014, 47, 1127‒1136. |
| [2] | T. Tsukamoto, T. Kambe, T. Imaoka, K. Yamamoto, Nat. Rev. Chem. 2021, 5, 338‒347. |
| [3] | T. Moriai, T. Tsukamoto, M. Tanabe, T. Kambe, K. Yamamoto, Angew. Chem. Int. Ed. 2020, 59, 23051‒23055. |
| [4] | T. Moriai, T. Tsukamoto, K. Fukuhara, T. Imaoka, T. Kambe, K. Yamamoto, Nanoscale Adv. 2025, 7, 346‒353. |
- 【Contact】
- Laboratory for Chemistry and Life Science, Institute of Science Tokyo
- Tatsuya Moriai, Asst. Prof. Email: moriai.t.aa@m.titech.ac.jp
- Kimihisa Yamamoto, Prof. Email: yamamoto@res.titech.ac.jp
- Takane Imaoka, Assoc. Prof. Email: timaoka@res.titech.ac.jp
- Masataka Yoshida, Asst. Prof. Email: yoshida.m.bo@m.titech.ac.jp
- TEL: 045-924-5259
- FAX: 045-924-5261



